ESO201A: Thermodynamics
... stroke engine, Limitations of Carnot cycle for real-time engineering systems, Lecture #33 Analysis of Otto and diesel cycles and derivation of thermal efficiencies, compression ratio and cut-off ratio, concept of relative efficiency, analysis of dual cycle (as homework), Real cycles and some brief d ...
... stroke engine, Limitations of Carnot cycle for real-time engineering systems, Lecture #33 Analysis of Otto and diesel cycles and derivation of thermal efficiencies, compression ratio and cut-off ratio, concept of relative efficiency, analysis of dual cycle (as homework), Real cycles and some brief d ...
ME 433 Combustion Engine Systems
... (ii) Sketch pressure-volume (P-V) and temperature-entropy (T-S) diagrams for the Otto cycle. CLEARLY LABEL THE ENDPOINTS OF EACH PROCESS. Begin with the start of the compression stroke. (iii) Make a table that shows the sign (-ve, 0, +ve) of heat transfer, work transfer, and change in internal energ ...
... (ii) Sketch pressure-volume (P-V) and temperature-entropy (T-S) diagrams for the Otto cycle. CLEARLY LABEL THE ENDPOINTS OF EACH PROCESS. Begin with the start of the compression stroke. (iii) Make a table that shows the sign (-ve, 0, +ve) of heat transfer, work transfer, and change in internal energ ...
More Carnot Cycle March 4, 2010 Efficiency = W/Qin = Qin
... the direction of heat flow is always from higher to the lower temperature. The diagram shows a rod of a conducting material with cross sectional area A and length L. The left end of the rod is kept at constant temperature TH and the right end at lower temperature TC. The sides of the rod are perfect ...
... the direction of heat flow is always from higher to the lower temperature. The diagram shows a rod of a conducting material with cross sectional area A and length L. The left end of the rod is kept at constant temperature TH and the right end at lower temperature TC. The sides of the rod are perfect ...
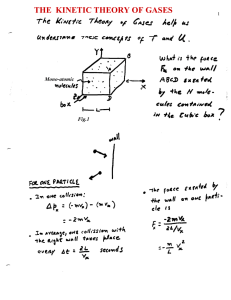
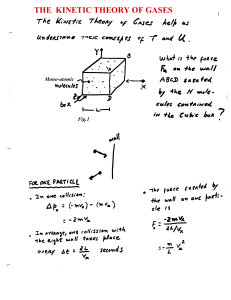
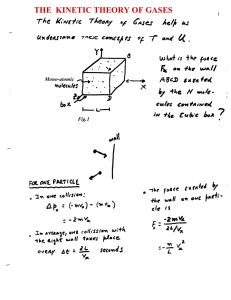
the kinetic theory of gases
... As an application of the expression above for the equation of state, let’s consider an adiabatic process, one in which the gas container is thermally well isolated, i.e. no heat-transfer occurs. No heat-transfer is added or removed to the gas, Q = 0 As the piston is pressed, work is done on the gas ...
... As an application of the expression above for the equation of state, let’s consider an adiabatic process, one in which the gas container is thermally well isolated, i.e. no heat-transfer occurs. No heat-transfer is added or removed to the gas, Q = 0 As the piston is pressed, work is done on the gas ...
Pot. Temp handout - Mechanical Engineering | University of Utah
... an adiabatic system, we know that a change in pressure results in a change in Temperature. This change must be accounted for when comparing displaced fluid parcels. b. The 1st Law of Thermodynamics Using the 1st Law of thermodynamics for an isentropic process (adiabatic and reversible), we can deter ...
... an adiabatic system, we know that a change in pressure results in a change in Temperature. This change must be accounted for when comparing displaced fluid parcels. b. The 1st Law of Thermodynamics Using the 1st Law of thermodynamics for an isentropic process (adiabatic and reversible), we can deter ...
CHAPTER I
... on the system and its internal energy is increased. If the piston moves out, then dV is positive, so W is positive and the system does work on its environment and its internal energy is reduced. This is a general expression of work for a gas, it isn’t piston and cylinder specific. For example, in a ...
... on the system and its internal energy is increased. If the piston moves out, then dV is positive, so W is positive and the system does work on its environment and its internal energy is reduced. This is a general expression of work for a gas, it isn’t piston and cylinder specific. For example, in a ...