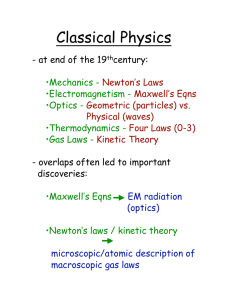
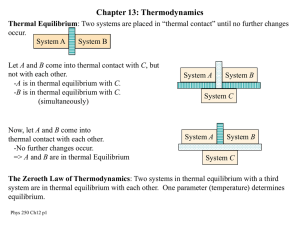
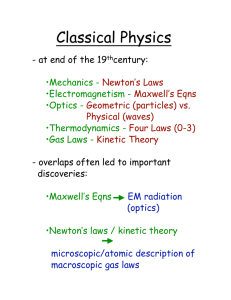
Kinetic Theory
... • the distribution of velocities for an ideal gas follows a Gaussian • the probability of a state is proportional to exp(-E/kT) • the entropy of an ideal gas led us to some familiar results from thermodynamics • we showed that for open systems the quantity G = E – TS is minimized ...
... • the distribution of velocities for an ideal gas follows a Gaussian • the probability of a state is proportional to exp(-E/kT) • the entropy of an ideal gas led us to some familiar results from thermodynamics • we showed that for open systems the quantity G = E – TS is minimized ...
PY2P10 Finn Problems Chap 1
... piston at the pressureP and volume I/. It is heatedquasistatically and at constantvolumeso that its temperatureis doubled,and then it is cooled at constant pressureuntil it returns to its original temperature.Show that the work done on the gasis PZ. 3.8 Find the change in the internal energy of one ...
... piston at the pressureP and volume I/. It is heatedquasistatically and at constantvolumeso that its temperatureis doubled,and then it is cooled at constant pressureuntil it returns to its original temperature.Show that the work done on the gasis PZ. 3.8 Find the change in the internal energy of one ...
metIstudyguide F14
... 11. What is dew point? 12. The hot seat belt against skin is what form of heat transfer? 13. Earth receives energy from sun through which method? 14. Heat flow that cycles (heat rises & cold sinks) is which form of heat transfer? 15. **Which cloud is… cotton-like & puffy=_____________________ Low & ...
... 11. What is dew point? 12. The hot seat belt against skin is what form of heat transfer? 13. Earth receives energy from sun through which method? 14. Heat flow that cycles (heat rises & cold sinks) is which form of heat transfer? 15. **Which cloud is… cotton-like & puffy=_____________________ Low & ...
15-2 Thermodynamic Processes and the First Law
... coffee and stir it, you wind up with coffee that is uniformly milky and sweet. No amount of stirring will get the milk and sugar to come back out of solution. ...
... coffee and stir it, you wind up with coffee that is uniformly milky and sweet. No amount of stirring will get the milk and sugar to come back out of solution. ...
Topic 2 The first law of thermodynamics
... The system is always infinitesimally close to equilibrium, and an infinitesimal change in conditions can reverse the process to restore both system and surrounding to their initial state. A reversible process is obviously an idealization Reversible isothermal process in a perfect gas ...
... The system is always infinitesimally close to equilibrium, and an infinitesimal change in conditions can reverse the process to restore both system and surrounding to their initial state. A reversible process is obviously an idealization Reversible isothermal process in a perfect gas ...