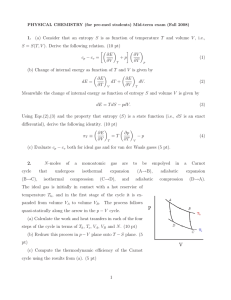
Section 11
... can be done by a gas in the cylinder if the gas exerts a constant pressure of 7.5 x 105 Pa on the piston and moves the piston a distance of 0.040 m? ...
... can be done by a gas in the cylinder if the gas exerts a constant pressure of 7.5 x 105 Pa on the piston and moves the piston a distance of 0.040 m? ...
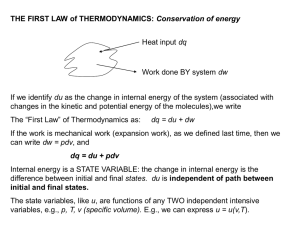
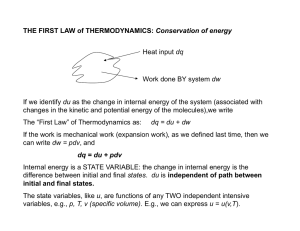
THE FIRST LAW of THERMODYNAMICS: Conservation of energy
... Since h = u + Pv and, for an ideal gas, Pv = RT, then for the ideal gas h = u + RT R is constant, and u = u(T) only, so we conclude h = h(T) only for an ideal gas. Aside: application of enthalpy balance in steady-flow system: energy balance for a single-stream, steady-flow system with negligible ch ...
... Since h = u + Pv and, for an ideal gas, Pv = RT, then for the ideal gas h = u + RT R is constant, and u = u(T) only, so we conclude h = h(T) only for an ideal gas. Aside: application of enthalpy balance in steady-flow system: energy balance for a single-stream, steady-flow system with negligible ch ...
Phases of Pure Substance
... Adiabatic Processes. No heat flows into or out of the system Qin 0 Adiabatic process then Eint Won,adiabatic n cV T The equation of curve describing the adiabatic process is ...
... Adiabatic Processes. No heat flows into or out of the system Qin 0 Adiabatic process then Eint Won,adiabatic n cV T The equation of curve describing the adiabatic process is ...