* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PPT version
Heat capacity wikipedia , lookup
Internal energy wikipedia , lookup
Van der Waals equation wikipedia , lookup
Calorimetry wikipedia , lookup
Thermal comfort wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Thermal conductivity wikipedia , lookup
Thermal radiation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Black-body radiation wikipedia , lookup
Heat transfer physics wikipedia , lookup
Heat transfer wikipedia , lookup
Equation of state wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Thermal expansion wikipedia , lookup
State of matter wikipedia , lookup
Thermoregulation wikipedia , lookup
Thermal conduction wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Adiabatic process wikipedia , lookup
Temperature wikipedia , lookup
Thermodynamic temperature wikipedia , lookup
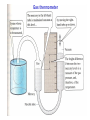
Thermodynamics. Study of temperature, heat and related macroscopic properties of objects and matter. Specifically macroscopic properties: temperature, pressure, heat, internal energy… Thermodynamic equilibrium: two systems are in thermodynamic equilibrium with each other, when they are in thermal contact and no change in any macroscopic property occurs with time. What do we mean by thermal contact? “Any macroscopic property” – length, volume, pressure, electrical conductivity, color. Why do we stress that we talk about MACROSCOPIC properties? Two systems have the same temperature if they are in thermodynamic equilibrium. Temperature is the parameter that two systems in thermodynamic equilibrium have in common. Zeroth law of thermodynamics: if two systems A and C each are in thermodynamic equilibrium with system B, then A and C are in thermodynamic equilibrium with each other. Sounds trivial, but gives a rationale for measuring temperature. Zeroth law of thermodynamics: if two systems A and C each are in thermodynamic equilibrium with system B, then A and C are in thermodynamic equilibrium with each other. B A C Liquid thermometers Based on thermal expansion of liquids. The liquids are held in little vessels at the bottom. At higher temperatures they expand and climb toward the top of narrow capillaries. How to calibrate a liquid thermometer? You need some reference temperatures. You may want to stick the thermometer into boiling water and melting ice, hoping that those set two well-defined temperatures. You will find the liquid expanded and its column risen. What next? We can divide the column height difference into N (say, N = 100) even intervals and call them degrees. Would it be a good temperature scale? Would it be the same, no matter what liquid we use? Liquid thermometers Coefficients of thermal expansion V / V T – fractional increase of volume per one degree Problems: 1. Liquid is a rather complex state of matter. Molecules in liquids are held together by cohesive forces, different for different liquids. 2. The coefficients of thermal expansion vary a lot between liquids, and may depend on temperature in an extreme fashion. 3. Liquids freeze at low temperatures and boil at high temperature. So the ranges of operation of the liquid thermometers are restricted. Gas thermometer Basically, a gas thermometer is like a barometer measuring absolute pressure of a gas in a closed volume. Gas thermometer Gasses are much simpler than liquids. The molecules are moving freely most of the time, and only once in a while suffer short term collisions. The collision events are still different for different molecules…. BUT: when the gasses are rarified (low density) and the collisions are rare the behavior of different gasses in the gas thermometer becomes very much the same! In order not to change the gas density, it is preferable to keep the gas volume constant and to measure the gas pressure. Gas thermometer Level of the mercury in the right tube is varied to keep the level in the left tube constant. Pressure of the gas is measured as P =rgh The absolute temperature of the system is defined as: P T 273.16 P3 P3 is pressure of the gas at a special reference point called “triple point”, which is unique and can be reproduced in every laboratory. Gas thermometer To set the temperature scale we need some convenient reference points. 273.15 K, the same as 0 °C is the temperature of ice melting at normal pressure; 373.15 K, the same as 100 °C is the temperature of water boiling at normal pressure; 1K temperature difference is the same as 1 °C temperature difference, but 0 K corresponds to -273.15 °C. Temperature scales Fahrenheit Celsius Lord Kelvin 9 TF 32 TC 5 TK 273.15 TC Thermometers: summary. 1. Thermometers always measure their own temeparature. 2. For a thermometer to measure the temperature of the system of interest, it needs to be brought into thermal equilibrium with the system (and better insulated from everything else). • A thermometer must be much smaller than system. • For fast temperature measurements, it should be small, have good thermal conductivity and low heat capacity. An array of miniature thermometers An array of bolometers Heat is not a material or a from of matter. Heat is energy in transit! When pouring water you transfer it from one vessel to another and you get more water in the second vessel. In thermodynamics you transfer heat but you usually end up having more or less internal energy. Heat is positive when the system of interest obtains it. What are common results of heat transfer? 1. Growth of temperature. 2. A phase transition (melting ice). 3. Mechanical work. In cases #2 and #3 there may be NO temperature variation. Case #1, no phase transition or work done. How much does the temperature vary? Heat capacity of the object C, measured in J/K; tells you how much Joules of heat you need to transfer to increase the temperature of the object by 1 K (or 1 ºC). Q C T Q CT Heat is energy in transit! Positive, when obtained.