Chapter 37 Wave Optics (I)
... Calculate the spacing between the bright fringes of yellow light of wavelength 600 nm. The slit separation is 0.8 mm, and the screen is 2 m from the slits. ...
... Calculate the spacing between the bright fringes of yellow light of wavelength 600 nm. The slit separation is 0.8 mm, and the screen is 2 m from the slits. ...
Length poster
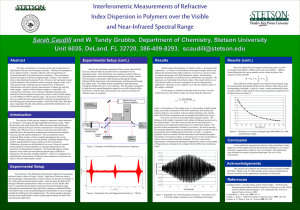
... (or vacuum wavelength), it is already possible to build lasers with an optical frequency that is known around ten thousand times more accurately. The iodinestabilised laser is limited because the iodine molecules are at room temperature and bang into each other, whizzing in and out of the laser beam ...
... (or vacuum wavelength), it is already possible to build lasers with an optical frequency that is known around ten thousand times more accurately. The iodinestabilised laser is limited because the iodine molecules are at room temperature and bang into each other, whizzing in and out of the laser beam ...
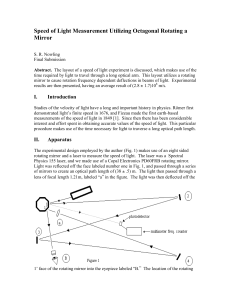
Speed of Light Measurement Utilizing Octagonal
... after the rotating mirror no shifts will ever be seen. This is because the shifts are so small that they are well within the paraxial approximation. The lens therefore sends all of the ÒparallelÓ rays to the same point, defeating the purpose of the entire experiment. As the mirror rotates the laser ...
... after the rotating mirror no shifts will ever be seen. This is because the shifts are so small that they are well within the paraxial approximation. The lens therefore sends all of the ÒparallelÓ rays to the same point, defeating the purpose of the entire experiment. As the mirror rotates the laser ...
Caffeine 200 mg Tablet Structure: Molecular Formula and Mass
... The high limit is 115%; therefore the concentration of the high standard = (0.200 mg/mL X 1.15 = 0.230 mg/mL. Weigh approximately 23.0 mg of standard. If you weighed 23.2 mg of standard, dissolve it in: (23.2 mg)/(0.230 mg/mL) = 101 mL of methanol. This makes the high standard solution concentration ...
... The high limit is 115%; therefore the concentration of the high standard = (0.200 mg/mL X 1.15 = 0.230 mg/mL. Weigh approximately 23.0 mg of standard. If you weighed 23.2 mg of standard, dissolve it in: (23.2 mg)/(0.230 mg/mL) = 101 mL of methanol. This makes the high standard solution concentration ...
Suman-AE-AOTFIntro-2..
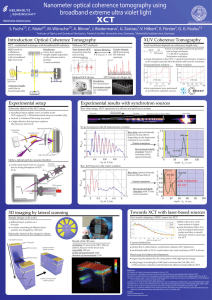
... applied RF. As indicated in the figure, the diffracted light intensity is directed into two first order beams, termed the (+) and (-) beams. These beams are orthogonally polarized, which is utilized in certain applications. To use the AOTF as a tunable filter, a beam stop is used to block the undiff ...
... applied RF. As indicated in the figure, the diffracted light intensity is directed into two first order beams, termed the (+) and (-) beams. These beams are orthogonally polarized, which is utilized in certain applications. To use the AOTF as a tunable filter, a beam stop is used to block the undiff ...
... Reflection: the incident and exit angle with respect to the normal to the surface are identical. Transmission: the refractive index change from one material to the next determines the change in direction from outside to inside the medium. Scattering: on a rough surface, locally the surface normal va ...
Practical Laboratory #2: Emission Spectra 2
... • measure the emission spectrum of a heated gas using the digital spectrometer. • record a number of the bright lines in the spectrum. • compare the measured spectrum with the known spectra for specific gases • identify the unknown gas. ...
... • measure the emission spectrum of a heated gas using the digital spectrometer. • record a number of the bright lines in the spectrum. • compare the measured spectrum with the known spectra for specific gases • identify the unknown gas. ...
Unit 5 Objectives
... 5. Given the mass of a substance, determine a. the number of moles of the sample b. the number of atoms or molecules in the sample ...
... 5. Given the mass of a substance, determine a. the number of moles of the sample b. the number of atoms or molecules in the sample ...
Chapter 29: Light Waves Interference Constructive Interference
... • The de Broglie hypothesis was unexpectedly experimentally confirmed in 1927 by two scientists firing electrons at a nickel crystal • The regular spacing between atoms in the crystal acts like a diffraction grating ...
... • The de Broglie hypothesis was unexpectedly experimentally confirmed in 1927 by two scientists firing electrons at a nickel crystal • The regular spacing between atoms in the crystal acts like a diffraction grating ...
supplemental problems
... a) What is the cutoff wavelength for this PMT? b) Will this PMT work in the visible portion of the spectrum? Why? Assume you are measuring 550 nm light at one point in the experiment and that 20 picoWatts of the light is incident upon the detector. The photocathode has a quantum efficiency of 22% at ...
... a) What is the cutoff wavelength for this PMT? b) Will this PMT work in the visible portion of the spectrum? Why? Assume you are measuring 550 nm light at one point in the experiment and that 20 picoWatts of the light is incident upon the detector. The photocathode has a quantum efficiency of 22% at ...
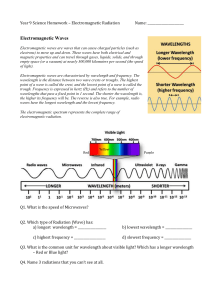
Chem 115 - Waves, Radiation and Spectroscopy (lecture 16) 3/31
... If you shine light of a particular wavelength onto metal you can get the electrons to come off of the metal if the light has high enough frequency (thus energy). The minimum threshold energy where electrons begin to come off the metal is called the “work function”. Photoelectric effect is an example ...
... If you shine light of a particular wavelength onto metal you can get the electrons to come off of the metal if the light has high enough frequency (thus energy). The minimum threshold energy where electrons begin to come off the metal is called the “work function”. Photoelectric effect is an example ...
n - Purdue Physics
... Primary colors for additive mixing: Cyan, Magenta, Yellow Cyan - absorbs red ...
... Primary colors for additive mixing: Cyan, Magenta, Yellow Cyan - absorbs red ...
Assignment #2 - Rose
... 3. (Saleh and Teich 3.2-1) An argon-ion laser produces a Gaussian beam of wavelength 488 nm and waist spot size of 0.5 mm. Design a single-lens optical system for focusing the light to a spot diameter of 100 m. What is the shortest focal-length lens that may be used? (There are two ways to approach ...
... 3. (Saleh and Teich 3.2-1) An argon-ion laser produces a Gaussian beam of wavelength 488 nm and waist spot size of 0.5 mm. Design a single-lens optical system for focusing the light to a spot diameter of 100 m. What is the shortest focal-length lens that may be used? (There are two ways to approach ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.