diffraction and interference
... constructive and fully destructive. Can have anything from 0 to 4 times as bright ...
... constructive and fully destructive. Can have anything from 0 to 4 times as bright ...
Chapter 24 Wave Optics Diffraction Grating Interference by Thin
... For a diffraction grating, the intensity falls away from these maxima much more rapidly than that for a double slit. Because there are so many slits to act as sources, any angle other than those for maxima will be dark or nearly dark. ...
... For a diffraction grating, the intensity falls away from these maxima much more rapidly than that for a double slit. Because there are so many slits to act as sources, any angle other than those for maxima will be dark or nearly dark. ...
Fiber Optic
... i) Responsitivity: Responsitivity is a measure of the conversion efficiency of a photodetector. ii ) Dark current: Dark current is the leakage current that flows through a photodiode with no light input. iii ) Transit time: Transit time is the time it takes a light-induced carrier to travel across t ...
... i) Responsitivity: Responsitivity is a measure of the conversion efficiency of a photodetector. ii ) Dark current: Dark current is the leakage current that flows through a photodiode with no light input. iii ) Transit time: Transit time is the time it takes a light-induced carrier to travel across t ...
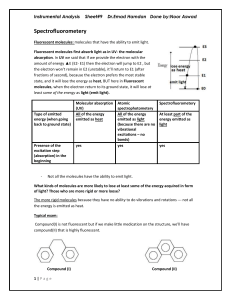
10.3.2.1.1 Spectral apparatus An optical arrangement or an
... arrangement or when the focal planes are different in the two dimensions, astigmatic. When the radiation passes through the same optical components before and after being dispersed, the spectral system is autocollimative. Spectral separation or isolation of optical radiation may be achieved by using ...
... arrangement or when the focal planes are different in the two dimensions, astigmatic. When the radiation passes through the same optical components before and after being dispersed, the spectral system is autocollimative. Spectral separation or isolation of optical radiation may be achieved by using ...
Bohr Model and EMS practice
... Use the Bohr Model of the Hydrogen Atom and the Electromagnetic Spectrum in the reference tables to answer the following questions: 1. When an electron in an excited state moves from n=6 to n=2, what wavelength of energy is emitted? What region of the EM spectrum is this wavelength located? 2. In wh ...
... Use the Bohr Model of the Hydrogen Atom and the Electromagnetic Spectrum in the reference tables to answer the following questions: 1. When an electron in an excited state moves from n=6 to n=2, what wavelength of energy is emitted? What region of the EM spectrum is this wavelength located? 2. In wh ...
VeeMAX UV-Visible Variable Angle Specular Reflectance Accessory
... • Mirrors operating at 45° on laser tables are required to direct the beam through experiments in research applications. • Hot mirrors, cold mirrors and bandpass mirrors have different cut-on and cut-off wavelengths depending on the angle of incidence. How does a shift from 30° to 45° affect your pr ...
... • Mirrors operating at 45° on laser tables are required to direct the beam through experiments in research applications. • Hot mirrors, cold mirrors and bandpass mirrors have different cut-on and cut-off wavelengths depending on the angle of incidence. How does a shift from 30° to 45° affect your pr ...
TAP 704- 7: Red shifts of quasars
... In 1962, Australian radio astronomers got an accurate position for one of the sources in the Third Cambridge Catalogue of radio sources. Its catalogue number was 3C273. They did it by watching 3C273 being eclipsed by the edge of the Moon. Optical telescopes picked it up as a very faint star-like obj ...
... In 1962, Australian radio astronomers got an accurate position for one of the sources in the Third Cambridge Catalogue of radio sources. Its catalogue number was 3C273. They did it by watching 3C273 being eclipsed by the edge of the Moon. Optical telescopes picked it up as a very faint star-like obj ...
HW #8 Solutions
... I’ve ever encountered a left-handed screw is on the left pedal of a bicycle, which uses such a screw so as not to unscrew as the pedal rotates.) Note that if you stand on the mirror and look down, your image will indeed be upside down. In this case, the ...
... I’ve ever encountered a left-handed screw is on the left pedal of a bicycle, which uses such a screw so as not to unscrew as the pedal rotates.) Note that if you stand on the mirror and look down, your image will indeed be upside down. In this case, the ...
Physical and Chemical Tests
... still not known with certainty. Many new substances are newly synthesized for the first time and their properties are not in the literature. ...
... still not known with certainty. Many new substances are newly synthesized for the first time and their properties are not in the literature. ...
Chapter 20 (Answers are all A`s) 1. Find the displacement of a
... 4. A simple harmonic wave described by the equation y(t) = 0.54 cos(3.1x – 2.3t) reflects from both ends of a string that is clamped at each end, resulting in a standing wave that is the sum of y(t) and and its reflection. What is the amplitude of the standing wave at x = 0.22 m? The quantities x an ...
... 4. A simple harmonic wave described by the equation y(t) = 0.54 cos(3.1x – 2.3t) reflects from both ends of a string that is clamped at each end, resulting in a standing wave that is the sum of y(t) and and its reflection. What is the amplitude of the standing wave at x = 0.22 m? The quantities x an ...
Chp.23 Outline - Redlands High School
... 1) What is a photon? Do all photons have the same energy? What is a quantum value? Give some examples of other quantum values besides the photon. Write the equation relating the frequency of a photon to its energy. Does a photon fit better in the particle or wave nature of light? What does an electr ...
... 1) What is a photon? Do all photons have the same energy? What is a quantum value? Give some examples of other quantum values besides the photon. Write the equation relating the frequency of a photon to its energy. Does a photon fit better in the particle or wave nature of light? What does an electr ...
Chapter 7 Worksheet November 1
... 5. A police officer uses a radar gun to catch Heisenberg speeding. The gun operates at a frequency of 22.235 x 10 9 Hz. Find the wavelength in nanometers of this radiation. (Bonus: What did Heisenberg say to the police officer when the police officer asked if he knew how fast he was going?) ...
... 5. A police officer uses a radar gun to catch Heisenberg speeding. The gun operates at a frequency of 22.235 x 10 9 Hz. Find the wavelength in nanometers of this radiation. (Bonus: What did Heisenberg say to the police officer when the police officer asked if he knew how fast he was going?) ...
Photoelectric Effect Practice Problems
... explain this contradiction by assuming that light energy is quantized and that higher frequencies of light have a bigger quantum. Explain why light energy being quantized this way means that a blackbody will emit less energy at higher frequencies. ...
... explain this contradiction by assuming that light energy is quantized and that higher frequencies of light have a bigger quantum. Explain why light energy being quantized this way means that a blackbody will emit less energy at higher frequencies. ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.