In this lab you will use the phenomenon of interference... thickness of thin films. Two interference techniques, Michelson and... Thin Film Measurement 1 Introduction
... Two measuring techniques are used and compared with this set-up. The first involves using the micrometer on the mirror to measure the difference in zero path length between the thin film and no film. The second technique uses the step height change in the interference pattern much like the Fizeau te ...
... Two measuring techniques are used and compared with this set-up. The first involves using the micrometer on the mirror to measure the difference in zero path length between the thin film and no film. The second technique uses the step height change in the interference pattern much like the Fizeau te ...
4-1. 1 - Riverside Local Schools
... the atom’s electron with… 2. According to the model, electrons can circle the nucleus only in 3. The electron is in its lowest energy state when it is in the… 4. The energy of the electron is higher when it is in orbits that are successively farther from the… 5. An electron can be in one orbit or an ...
... the atom’s electron with… 2. According to the model, electrons can circle the nucleus only in 3. The electron is in its lowest energy state when it is in the… 4. The energy of the electron is higher when it is in orbits that are successively farther from the… 5. An electron can be in one orbit or an ...
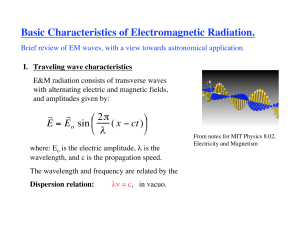
A solution to Maxwell`s equations in free space
... In a wave, the fields change with time. Therefore the Poynting vector changes too!!The direction is constant, but the magnitude changes from 0 to a maximum ...
... In a wave, the fields change with time. Therefore the Poynting vector changes too!!The direction is constant, but the magnitude changes from 0 to a maximum ...
PHYSICS 113 Assignment #3 SOLUTIONS Chapter 4 19. How many
... 7. Why do astronomers place telescopes in Earth orbit? What are the advantages for different spectral regions? Astronomers place telescopes in Earth orbit in order to observe radiation from those parts of the EM spectrum for which the atmosphere would normally absorb or significantly scatter that ra ...
... 7. Why do astronomers place telescopes in Earth orbit? What are the advantages for different spectral regions? Astronomers place telescopes in Earth orbit in order to observe radiation from those parts of the EM spectrum for which the atmosphere would normally absorb or significantly scatter that ra ...
Lab 11: Atomic Spectra
... Part 4: Fluorescence Fluorescence occurs when an atom absorbs light at one frequency but then emits light at lower frequencies. For example, an atom can absorb a UV photon and jump to a higher energy state. Rather then jumping directly back to the initial energy state (and emitting the same energy ...
... Part 4: Fluorescence Fluorescence occurs when an atom absorbs light at one frequency but then emits light at lower frequencies. For example, an atom can absorb a UV photon and jump to a higher energy state. Rather then jumping directly back to the initial energy state (and emitting the same energy ...
Measurement of the Wavelength by Diffraction Gratings
... in Young’s double slit experiment where the image is wide and the colors are mixed. As a matter of fact a diffraction grating analysis the light to its component colors very well (better than a prism). ...
... in Young’s double slit experiment where the image is wide and the colors are mixed. As a matter of fact a diffraction grating analysis the light to its component colors very well (better than a prism). ...
Lecture 16 - Purdue Physics
... diverging from that point. The rays propagate in straight lines until they encounter an interface to another type of material. This defines the ray model of light. ...
... diverging from that point. The rays propagate in straight lines until they encounter an interface to another type of material. This defines the ray model of light. ...
The University of Georgia Department of Physics and Astronomy
... Mach-Zender interferometer shown in the figure. Light from a infrared laser (λ = 1.00µm) is split into two waves that travel equal distances around the arms of the interferometer. One arm passes through an electro-optic crystal, a transparent material that can change its index of refraction in respo ...
... Mach-Zender interferometer shown in the figure. Light from a infrared laser (λ = 1.00µm) is split into two waves that travel equal distances around the arms of the interferometer. One arm passes through an electro-optic crystal, a transparent material that can change its index of refraction in respo ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.