Relationship between the electric field and magnetic field
... does some work on the charge (i.e. deposit some energy W), and, as a consequence, the charge feels some force F The example above suggests: ...
... does some work on the charge (i.e. deposit some energy W), and, as a consequence, the charge feels some force F The example above suggests: ...
Optical Properties of Condensed Matters
... The molecular materials are held together by the weak van de Waals bonds, whereas the molecules are held together by strong covalent bonds. The optical properties of materials are similar to those of the individual molecules; Saturated compounds: compounds which do not contain any free valence (all ...
... The molecular materials are held together by the weak van de Waals bonds, whereas the molecules are held together by strong covalent bonds. The optical properties of materials are similar to those of the individual molecules; Saturated compounds: compounds which do not contain any free valence (all ...
Activity 2 - hrsbstaff.ednet.ns.ca
... (b) Determine the time taken for the photons to travel 0.30 m from the filters to the detector. (c) Each filter absorbs 96% of the photons. How many photons per second pass through after seven filters? (d) Compare the time taken by each photon to travel 0.30 m with the time between successive photon ...
... (b) Determine the time taken for the photons to travel 0.30 m from the filters to the detector. (c) Each filter absorbs 96% of the photons. How many photons per second pass through after seven filters? (d) Compare the time taken by each photon to travel 0.30 m with the time between successive photon ...
Quantitative Analysis Spectroscope #CQ$ 42581
... that travels in small particle like packets called photons. Each photon travels at the same speed: 3 x 10s m/see, the speed of light. The energy era photon is determined by its frequency (color). The higher the frequency, the more energy it contains. The higher the frequency, the bluer the light, th ...
... that travels in small particle like packets called photons. Each photon travels at the same speed: 3 x 10s m/see, the speed of light. The energy era photon is determined by its frequency (color). The higher the frequency, the more energy it contains. The higher the frequency, the bluer the light, th ...
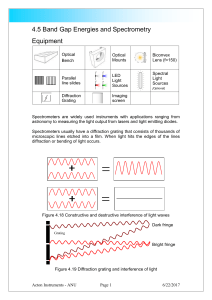
4.5 Band Gap Energies and Spectrometry
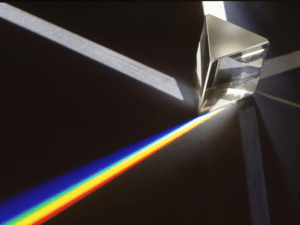
... The amount of bending depends on the wavelength or colour of the light. The light can constructively or destructively interfere to leave areas of darkness and colour. The amount of bending depends on the spacing and size of the lines on the diffraction grating. Light can be produced in a number of ...
... The amount of bending depends on the wavelength or colour of the light. The light can constructively or destructively interfere to leave areas of darkness and colour. The amount of bending depends on the spacing and size of the lines on the diffraction grating. Light can be produced in a number of ...
Unit Study Guide - Lighthouse Christian Academy
... bioluminescence – living things which can make themselves luminous using a chemical reaction. Chemiluminescence – the process of changing chemical energy into light energy with little or no change in temperature crest - the highest point on a wave; on a graph it is the farthest point above the x-axi ...
... bioluminescence – living things which can make themselves luminous using a chemical reaction. Chemiluminescence – the process of changing chemical energy into light energy with little or no change in temperature crest - the highest point on a wave; on a graph it is the farthest point above the x-axi ...
2.2.3.- X-ray diffraction
... Moreover, the time for step was selected to be relatively long (10 to 20 s) in order to reduce the statistical error. The powder diffractometer used in our study was set up in a Bragg-Brentano geometry (see fig. 2.4). In this geometry, both the x-ray source and the detector are located in the focali ...
... Moreover, the time for step was selected to be relatively long (10 to 20 s) in order to reduce the statistical error. The powder diffractometer used in our study was set up in a Bragg-Brentano geometry (see fig. 2.4). In this geometry, both the x-ray source and the detector are located in the focali ...
Lecture 28 - LSU Physics
... A thickness D=(m+0.5) 2.02 µm would make the waves OUT of phase. For example, 1.008 mm makes them in phase, and 1.010 mm makes them OUT of phase. ...
... A thickness D=(m+0.5) 2.02 µm would make the waves OUT of phase. For example, 1.008 mm makes them in phase, and 1.010 mm makes them OUT of phase. ...
Lecture 33 : Chiral molecules and Optical Activity
... Similar to rotation of plane of polarized light, one can have selective absorption of light depending on how molecules are oriented with respect to it. This phenomenon, involving anisotropic absorption of polarized light, is known as dichroism. For solids or liquid crystals we use the term linear di ...
... Similar to rotation of plane of polarized light, one can have selective absorption of light depending on how molecules are oriented with respect to it. This phenomenon, involving anisotropic absorption of polarized light, is known as dichroism. For solids or liquid crystals we use the term linear di ...
IOSR Journal of Applied Physics (IOSR-JAP)
... From the figure 7, it was found that linear plot intercepted the photon energy axis at 1.35eV and this value is taken to be the band gap energy of the material deposited at 24hrs. The band gap energy was also determined for sample 2(48hrs). The linear plot intercepted the photon energy axis at 1.25e ...
... From the figure 7, it was found that linear plot intercepted the photon energy axis at 1.35eV and this value is taken to be the band gap energy of the material deposited at 24hrs. The band gap energy was also determined for sample 2(48hrs). The linear plot intercepted the photon energy axis at 1.25e ...
Statistics and chemometrics for analytical chemistry
... J. N. Miller Other References Course Description: ...
... J. N. Miller Other References Course Description: ...
Culver City H.S. • AP Chemistry Name Period ___ Date ___/___/___
... An electron is excited from the n=1 ground state to the n=3 state in a hydrogen atom. Which of the following statements are true? Correct the false statements to make them true. It takes more energy to ionize (completely remove) the electron from n=3 than from the ground state. The electron is farth ...
... An electron is excited from the n=1 ground state to the n=3 state in a hydrogen atom. Which of the following statements are true? Correct the false statements to make them true. It takes more energy to ionize (completely remove) the electron from n=3 than from the ground state. The electron is farth ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.