THE PHOTOELECTRIC EFFECT
... The phototube uses an emitter made of potassium metal. The accepted value for the work function of potassium is 2.24 eV, but there are other sources of voltage in the experiment, such as contact potentials of dissimilar metals, that may distort this value. The collector is a circular wire constructe ...
... The phototube uses an emitter made of potassium metal. The accepted value for the work function of potassium is 2.24 eV, but there are other sources of voltage in the experiment, such as contact potentials of dissimilar metals, that may distort this value. The collector is a circular wire constructe ...
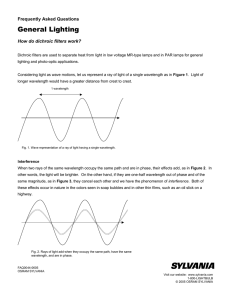
Interference 1 - schoolphysics
... the radio reception varies in amplitude, maxima occurring every 10 m within the first 200 m above the sea. If the transmitter is 150 m above sea level explain this effect and calculate the wavelength of the transmitted radio signal. 6. A Young’s double slit experiment is carried out using green ligh ...
... the radio reception varies in amplitude, maxima occurring every 10 m within the first 200 m above the sea. If the transmitter is 150 m above sea level explain this effect and calculate the wavelength of the transmitted radio signal. 6. A Young’s double slit experiment is carried out using green ligh ...
Chapter 4 Questions Perception of Color
... • The atmosphere refracts and scatters light into our eyes even after the sun has set. ...
... • The atmosphere refracts and scatters light into our eyes even after the sun has set. ...
158 The components of light
... electronic shell of an atom consists of certain, well-defined orbitals. However, the shell can also be decomposed is a different way, and this is indeed done, when it is convenient. These parts or contributions is then given the somewhat daunting name hybrid orbitals. To the student it appears as so ...
... electronic shell of an atom consists of certain, well-defined orbitals. However, the shell can also be decomposed is a different way, and this is indeed done, when it is convenient. These parts or contributions is then given the somewhat daunting name hybrid orbitals. To the student it appears as so ...
Chapter8_notes
... used for quantitative analysis. • Radiationless transitions can occur resulting in a Stoke’s Shift (longer fluorescence line). ...
... used for quantitative analysis. • Radiationless transitions can occur resulting in a Stoke’s Shift (longer fluorescence line). ...
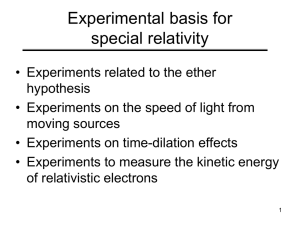
Experimental basis for special relativity
... • Change in the apparent position of a star due to changes in the velocity of the earth in its orbit • Fresnel attempted to explain this from a theory of the velocity of light in a moving medium • According to Fresnel, the ether was dragged along with the earth and this gave rise to the aberration e ...
... • Change in the apparent position of a star due to changes in the velocity of the earth in its orbit • Fresnel attempted to explain this from a theory of the velocity of light in a moving medium • According to Fresnel, the ether was dragged along with the earth and this gave rise to the aberration e ...
type worksheet title here
... 3) When 60.0 milliliter of 3.0-molar K2CO3 is added to 40.0 milliliters of 1.0-molar KHCO3 the resulting concentration of K+ is ...
... 3) When 60.0 milliliter of 3.0-molar K2CO3 is added to 40.0 milliliters of 1.0-molar KHCO3 the resulting concentration of K+ is ...
Huygens` and Fermat`s Principles – Application to reflection
... taken by a beam of light is the one that is traversed in the least time” ...
... taken by a beam of light is the one that is traversed in the least time” ...
L 35 Modern Physics [1] Modern Physics
... • A certain amount of energy is required to make an electron pop out of a metal • A photoelectron is emitted if it absorbs a photon from the light beam that has enough energy (high enough frequency) • No matter how many photons hit the electron, if they don’t have the right frequency the electron do ...
... • A certain amount of energy is required to make an electron pop out of a metal • A photoelectron is emitted if it absorbs a photon from the light beam that has enough energy (high enough frequency) • No matter how many photons hit the electron, if they don’t have the right frequency the electron do ...
L 35 Modern Physics [1]
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
Chem 2 AP Ch 7 MC Review Key
... 1. Some copper compounds emit green light when they are heated in a flame. How would you determine whether the light is of one wavelength or a mixture of two or more wavelengths? A) Observe the emitted light with green tinted glasses. B) Pass the emitted light through a beaker of water. C) Pass the ...
... 1. Some copper compounds emit green light when they are heated in a flame. How would you determine whether the light is of one wavelength or a mixture of two or more wavelengths? A) Observe the emitted light with green tinted glasses. B) Pass the emitted light through a beaker of water. C) Pass the ...
Presentation
... In 1909 G.I. Taylor experimented with a very dim light source. His work, and many modern experiments show that even though only one photon passes through a double slit, over time, an interference pattern is still produced one “particle” at a time. =h/p “It would seem that the basic idea of the qua ...
... In 1909 G.I. Taylor experimented with a very dim light source. His work, and many modern experiments show that even though only one photon passes through a double slit, over time, an interference pattern is still produced one “particle” at a time. =h/p “It would seem that the basic idea of the qua ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.












![L 35 Modern Physics [1] - University of Iowa Physics](http://s1.studyres.com/store/data/000679677_1-b925cf8c8f031b0f2b0c09a806312d20-300x300.png)

![L 35 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/001558975_1-84d6e03bc786b63795533f59711ce2f4-300x300.png)
![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/001689016_1-3e506855e2f70cb00e132a79d00855e2-300x300.png)