The Spring 2006 Qualifying Exam, Part 1
... 2. The rate of photoelectron emission is proportional to the light intensity but does not depend on its frequency. 3. The energy of the emitted electron varies linearly with the frequency of the light but is independent of its intensity. 4. There is a threshold frequency for photoemission that is in ...
... 2. The rate of photoelectron emission is proportional to the light intensity but does not depend on its frequency. 3. The energy of the emitted electron varies linearly with the frequency of the light but is independent of its intensity. 4. There is a threshold frequency for photoemission that is in ...
THE PHOTOELECTRIC EFFECT
... The phototube uses an emitter made of potassium metal. The accepted value for the work function of potassium is 2.24 eV, but there are other sources of voltage in the experiment, such as contact potentials of dissimilar metals, that may distort this value. The collector is a circular wire constructe ...
... The phototube uses an emitter made of potassium metal. The accepted value for the work function of potassium is 2.24 eV, but there are other sources of voltage in the experiment, such as contact potentials of dissimilar metals, that may distort this value. The collector is a circular wire constructe ...
L 35 Modern Physics [1]
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
Birla Institute of Technology and Science, Pilani and Elite School of Optometry
... COURSE HANDOUT COURSE NO. ...
... COURSE HANDOUT COURSE NO. ...
Light and Quantized Energy
... the same info. You need to include everything except the wavelength and frequency numbers. Also, since this diagram is not in color, either color or label the colors of the visible light spectrum (no indigo). ...
... the same info. You need to include everything except the wavelength and frequency numbers. Also, since this diagram is not in color, either color or label the colors of the visible light spectrum (no indigo). ...
Chemistry Learning Goals Chap 14 Solutions Minniear
... SWBAT define miscible and immiscible and given examples of each. SWBAT define solvent and solute and in a given solution, identify the solute and solvent. SWBAT explain saturated, unsaturated and supersaturated solutions. SWBAT explain the processes involved in the dissolving mechanism (dissociation ...
... SWBAT define miscible and immiscible and given examples of each. SWBAT define solvent and solute and in a given solution, identify the solute and solvent. SWBAT explain saturated, unsaturated and supersaturated solutions. SWBAT explain the processes involved in the dissolving mechanism (dissociation ...
Common Lighting Terminology Ambient Light The light already
... Light from an ordinary light bulb containing a thin coiled tungsten wire that becomes incandescent (emits light) when an electric current is passed along it. Tungsten colour temperature is around 2800K to 3400K. Also known as incandescent light. ...
... Light from an ordinary light bulb containing a thin coiled tungsten wire that becomes incandescent (emits light) when an electric current is passed along it. Tungsten colour temperature is around 2800K to 3400K. Also known as incandescent light. ...
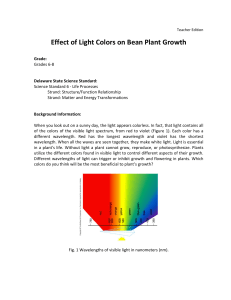
Effect of Light Colors on Bean Plant Growth
... ensure that at least one grows. After they sprout, snip one of them off so that there will be one per cup. This project will take several weeks to measure plant growth. The beans should sprout within a few days. During this experiment, explain figure 1 - that the line graph indicates the wavelengths ...
... ensure that at least one grows. After they sprout, snip one of them off so that there will be one per cup. This project will take several weeks to measure plant growth. The beans should sprout within a few days. During this experiment, explain figure 1 - that the line graph indicates the wavelengths ...
Semiconductor Devices
... If light of the proper wavelength is incident on the depletion region of a diode while a reverse voltage is applied, the absorbed photons can produce additional electron-hole pairs. This is photoconduction and many photocells are based on this property. ...
... If light of the proper wavelength is incident on the depletion region of a diode while a reverse voltage is applied, the absorbed photons can produce additional electron-hole pairs. This is photoconduction and many photocells are based on this property. ...
CENTENNIAL HONORS COLLEGE Western Illinois University Undergraduate Research Day 2016
... It has been known that under sunlight the external shell of many beetles in the scarabaeidae family reflects only left-handed circularly polarized light. Light is a transverse electromagnetic wave. When viewed toward the light source, the end of the electric field may t ...
... It has been known that under sunlight the external shell of many beetles in the scarabaeidae family reflects only left-handed circularly polarized light. Light is a transverse electromagnetic wave. When viewed toward the light source, the end of the electric field may t ...
The electromagnetic spectrum
... The speed of sound is 0.2 miles / second. How long does it take the sound of a lightning strike to reach you if it occurs 0.2 miles away? A. 0.2 seconds B. 1 second C. 5 seconds D. 20 seconds ...
... The speed of sound is 0.2 miles / second. How long does it take the sound of a lightning strike to reach you if it occurs 0.2 miles away? A. 0.2 seconds B. 1 second C. 5 seconds D. 20 seconds ...
LIGHT
... If you said black you were right. Even though all the wavelengths of visible white light strike the car’s surface, only the blue wavelengths are reflected by the pigments in the paint. Since the blue wavelengths are blocked by the red filter, no light waves from the car can reach your eyes, so the ...
... If you said black you were right. Even though all the wavelengths of visible white light strike the car’s surface, only the blue wavelengths are reflected by the pigments in the paint. Since the blue wavelengths are blocked by the red filter, no light waves from the car can reach your eyes, so the ...
METO 621
... • A quantum of radiative energy is called a photon, and is given the symbol hn . Hence in a chemical equation we write: O3 + hn →O2 + O • The energy of a photon in terms of its wavelength l is E=119625/l kJ mol-1 or 1239.8/ l in eV • To get enough energy to break up a molecule (dissociation) the wav ...
... • A quantum of radiative energy is called a photon, and is given the symbol hn . Hence in a chemical equation we write: O3 + hn →O2 + O • The energy of a photon in terms of its wavelength l is E=119625/l kJ mol-1 or 1239.8/ l in eV • To get enough energy to break up a molecule (dissociation) the wav ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.




![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/000572764_1-c4bf5ed66474525e3cf4981a43e1bbe1-300x300.png)