Chapter 35 Light: Reflection and Refraction
... Chapter 35 Light: Reflection and Refraction 35.1 Ray Optics It is natural to treat the propagation of light in terms of rays. A ray is equivalent to a very narrow beam of light, and it indicates the path along which the energy of the wave travels. Geometrical optics is the study of the behavior of s ...
... Chapter 35 Light: Reflection and Refraction 35.1 Ray Optics It is natural to treat the propagation of light in terms of rays. A ray is equivalent to a very narrow beam of light, and it indicates the path along which the energy of the wave travels. Geometrical optics is the study of the behavior of s ...
Topic 16: Geometric Optics
... Among Young’s varied interests was Egyptology. This led him, on the summer of 1814, to take up the study of the Rosetta Stone. Young’s breakthrough in deciphering hieroglyphics was simple. He assumed a group of encircled symbols or cartouche represented the Pharaoh Ptolemy and would have a similar p ...
... Among Young’s varied interests was Egyptology. This led him, on the summer of 1814, to take up the study of the Rosetta Stone. Young’s breakthrough in deciphering hieroglyphics was simple. He assumed a group of encircled symbols or cartouche represented the Pharaoh Ptolemy and would have a similar p ...
Unit 13: EM Radiation and Waves
... Why Clouds are Wight? • Clouds are white because their water droplets or ice crystals are large enough to scatter the light of the seven wavelengths which combine to produce white light. • Clouds will appear dark or gray when either they are in another clouds shadow or the top of a cloud casts a sh ...
... Why Clouds are Wight? • Clouds are white because their water droplets or ice crystals are large enough to scatter the light of the seven wavelengths which combine to produce white light. • Clouds will appear dark or gray when either they are in another clouds shadow or the top of a cloud casts a sh ...
Work sheet –chapter 2 CLASS - XI CHEMISTRY (Structure of Atom
... 5. What did Einstein explain about photoelectric effect? 6. What is the relation between kinetic energy and frequency of the photoelectrons? 7. Calculate energy of 2mole of photons of radiation whose frequency is 51014Hz. 8. What is emission and absorption spectra? 9. What transition in the hydroge ...
... 5. What did Einstein explain about photoelectric effect? 6. What is the relation between kinetic energy and frequency of the photoelectrons? 7. Calculate energy of 2mole of photons of radiation whose frequency is 51014Hz. 8. What is emission and absorption spectra? 9. What transition in the hydroge ...
Optics-Light Lab - University of Michigan SharePoint Portal
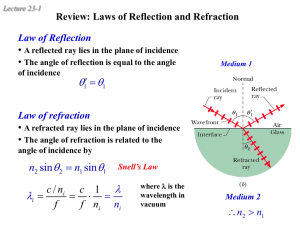
... 1. The law of reflection states that the angle of reflection is equal to the angle of incidence for a reflected ray of light. The angles are defined with respect to the direction perpendicular to the reflecting surface called the normal. 2. Concave mirrors focus rays so that they converge at a commo ...
... 1. The law of reflection states that the angle of reflection is equal to the angle of incidence for a reflected ray of light. The angles are defined with respect to the direction perpendicular to the reflecting surface called the normal. 2. Concave mirrors focus rays so that they converge at a commo ...
Document
... the reflectivity as far as possible. One very effective way of reducing the reflectivity of a glass surface is to coat it with an antireflection (AR) coating (giving an ARC). A good ARC can cut the percentage of light reflected from >5% to <0.2%. One example is the purple colored ARC on binocular an ...
... the reflectivity as far as possible. One very effective way of reducing the reflectivity of a glass surface is to coat it with an antireflection (AR) coating (giving an ARC). A good ARC can cut the percentage of light reflected from >5% to <0.2%. One example is the purple colored ARC on binocular an ...
WINDOWS During the Apollo (manned lunar exploration) space
... For parallel rays of visible light passing through a window, one medium probably will be air (n1 = 1.0). The other probably will be some other glass (n2 ~ 1.5). Using Equation 3, you can calculate a reflection of ~ 4%. This calculation is for one side of the window. When the rays emerge from the oth ...
... For parallel rays of visible light passing through a window, one medium probably will be air (n1 = 1.0). The other probably will be some other glass (n2 ~ 1.5). Using Equation 3, you can calculate a reflection of ~ 4%. This calculation is for one side of the window. When the rays emerge from the oth ...
Document
... • Long pathlength (10 cm) cells – used to study dilute (few molecules) or weakly absorbing samples. • Multipass cells – more compact and efficient instead of long-pathlength cells. Mirrors are used so that the beam makes several passes through the sample before exiting the cell. (Effective pathleng ...
... • Long pathlength (10 cm) cells – used to study dilute (few molecules) or weakly absorbing samples. • Multipass cells – more compact and efficient instead of long-pathlength cells. Mirrors are used so that the beam makes several passes through the sample before exiting the cell. (Effective pathleng ...
document
... two different media. This spreading of light is called chromatic dispersion. White light: It consists of components of nearly all the colors in the visible spectrum with approximately uniform intensities. The component of a beam of white light with shorter wavelength tends to be bent more. Spectrome ...
... two different media. This spreading of light is called chromatic dispersion. White light: It consists of components of nearly all the colors in the visible spectrum with approximately uniform intensities. The component of a beam of white light with shorter wavelength tends to be bent more. Spectrome ...
explanation
... How do we “see” things? It works like this. We need to expose an object to a source of light. If we are outside in the daylight, the light source is provided by the sun for free. When light hits the atoms the object is made of, various things can happen. The light can be absorbed, reflected or trans ...
... How do we “see” things? It works like this. We need to expose an object to a source of light. If we are outside in the daylight, the light source is provided by the sun for free. When light hits the atoms the object is made of, various things can happen. The light can be absorbed, reflected or trans ...
L 35 Modern Physics [1] Modern Physics
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
Spectroscopic methods for biology and medicine
... It is helpful, however, that a large part of biological molecules is constructed from only a small set of building blocks. 20 Amino acids form the molecular building blocks for peptides and proteins and are shown in Fig. 1.2. Bound by peptide bonds (-CO-NH-), amino acid chains are called peptides (m ...
... It is helpful, however, that a large part of biological molecules is constructed from only a small set of building blocks. 20 Amino acids form the molecular building blocks for peptides and proteins and are shown in Fig. 1.2. Bound by peptide bonds (-CO-NH-), amino acid chains are called peptides (m ...
Fourier transform infrared spectroscopy of aqueous solutions using
... For the analysis of small concentrations of organics in aqueous solutions, a novel add-on accessory for dualbeam / optical subtraction spectroscopy has been built for a commercial Fourier transform infrared spectrometer. A standard FT-JR instrument requires a sample measurement and a separate refere ...
... For the analysis of small concentrations of organics in aqueous solutions, a novel add-on accessory for dualbeam / optical subtraction spectroscopy has been built for a commercial Fourier transform infrared spectrometer. A standard FT-JR instrument requires a sample measurement and a separate refere ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.














![L 35 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/003926344_1-b779c05b753c6dc3972377c21f9bdcd3-300x300.png)