PHYS 113: Quantum Mechanics Waves and Interference In much of
... In much of our class, we have taken the view that everything in the world behaves more or less like a particle. Even photons, which we describe by their wavelength (note the “wave”) are said to have a particular position and velocity. We’ve spent very little time talking about the wavelike propertie ...
... In much of our class, we have taken the view that everything in the world behaves more or less like a particle. Even photons, which we describe by their wavelength (note the “wave”) are said to have a particular position and velocity. We’ve spent very little time talking about the wavelike propertie ...
Diffraction-of-light
... Optical effects resulting from diffraction are produced through the interference of light waves. To visualize this, imagine light waves as water waves. If water waves contact a float on the water surface, the float would bounce up and down in response to the oncoming waves, thenproducing more waves ...
... Optical effects resulting from diffraction are produced through the interference of light waves. To visualize this, imagine light waves as water waves. If water waves contact a float on the water surface, the float would bounce up and down in response to the oncoming waves, thenproducing more waves ...
Spectra of Underwater Light-Field Fluctuations in the Photic Zone
... This is in good agreement with the appearance of the sharp peak occurring in the 4-meter spectrum. There are, of course, other benefits to this kind of analysis of irradiance data which do not contribute to the biological application discussed above, but should be briefly discussed for the sake of c ...
... This is in good agreement with the appearance of the sharp peak occurring in the 4-meter spectrum. There are, of course, other benefits to this kind of analysis of irradiance data which do not contribute to the biological application discussed above, but should be briefly discussed for the sake of c ...
Chapter 24
... When charges vibrate, they act like tiny antennas. The electric field will oscillate in the direction of the vibration. Because vibration can occur in all directions, the resultant e-m wave is a superposition of the waves produced by the vibrating charges. This results in unpolarized light. If the ...
... When charges vibrate, they act like tiny antennas. The electric field will oscillate in the direction of the vibration. Because vibration can occur in all directions, the resultant e-m wave is a superposition of the waves produced by the vibrating charges. This results in unpolarized light. If the ...
Electron Configurations
... • Matter waves = wavelike behavior of particles. • Wave nature is inversely related to mass so we don’t notice wave nature of large objects. • However, electrons have a small mass and the wave characteristic is ...
... • Matter waves = wavelike behavior of particles. • Wave nature is inversely related to mass so we don’t notice wave nature of large objects. • However, electrons have a small mass and the wave characteristic is ...
Topic 5 Core Questions
... wave as it passes between different materials. It is caused by the slowing down or speeding up of the wave as it travels from one density to a different density. Away from the normal line. They will be parallel to each other. You might also notice the incident ray is slightly brighter than the emerg ...
... wave as it passes between different materials. It is caused by the slowing down or speeding up of the wave as it travels from one density to a different density. Away from the normal line. They will be parallel to each other. You might also notice the incident ray is slightly brighter than the emerg ...
Atomic Emission Spectrometry - San Diego Unified School District
... wavelengths that make it up. You can see spectra using a spectroscope, a prism or a diffraction grating. A spectroscope is a device which uses a diffraction grating to create a visual spectrum in a way that places the spectrum on a scale. This enables the user to measure the wavelengths of light bei ...
... wavelengths that make it up. You can see spectra using a spectroscope, a prism or a diffraction grating. A spectroscope is a device which uses a diffraction grating to create a visual spectrum in a way that places the spectrum on a scale. This enables the user to measure the wavelengths of light bei ...
Demonstration of Optical Rotatory Dispersion of Sucrose
... it propagates through an optically active solution. Linearly polarized light from a HeNe laser is directed down a 1-m cylindrical glass tube filled with a solution containing chiral molecules. Polarized light is scattered at right angles to the direction of propagation as a result of a combination o ...
... it propagates through an optically active solution. Linearly polarized light from a HeNe laser is directed down a 1-m cylindrical glass tube filled with a solution containing chiral molecules. Polarized light is scattered at right angles to the direction of propagation as a result of a combination o ...
Mathematical Methods of Physics – Fall 2010 – Dr
... photons have energy E = h = hc/ and momentum p = h/ can be created or destroyed when radiation (e.g. particles) are emitted or absorbed can have particle-like collisions with other particles such as electrons Light also exhibits wave-like properties such as interference and diffraction. QM: ...
... photons have energy E = h = hc/ and momentum p = h/ can be created or destroyed when radiation (e.g. particles) are emitted or absorbed can have particle-like collisions with other particles such as electrons Light also exhibits wave-like properties such as interference and diffraction. QM: ...
Monochromatic plane waves ( ) Plane waves have straight wave fronts
... in the least at the atomic scale. Yet full translational symmetry is an excellent approximation whenever the region is homogeneous on the scale of the light wavelength. ...
... in the least at the atomic scale. Yet full translational symmetry is an excellent approximation whenever the region is homogeneous on the scale of the light wavelength. ...
pptx
... A radio station transmits a 10 kW signal at a frequency of 100 MHz. (We will assume it radiates as a point source). At a distance of 1km from the antenna, find (a) the amplitude of the electric and magnetic field strengths, and (b) the energy incident normally on a square plate of side 10cm in 5min. ...
... A radio station transmits a 10 kW signal at a frequency of 100 MHz. (We will assume it radiates as a point source). At a distance of 1km from the antenna, find (a) the amplitude of the electric and magnetic field strengths, and (b) the energy incident normally on a square plate of side 10cm in 5min. ...
1: Inroduction
... effect. The source of the directional reflectance resides in the cone cells. These cells are mainly known for their sensitivity to light. They have an elongated cylindrical shape. In the healthy eye, the long axes of the cells are pointed precisely toward a common location near the center of the pup ...
... effect. The source of the directional reflectance resides in the cone cells. These cells are mainly known for their sensitivity to light. They have an elongated cylindrical shape. In the healthy eye, the long axes of the cells are pointed precisely toward a common location near the center of the pup ...
Visible Spectroscopy
... can be used to relate the frequency of light () to its wavelength (). Use 3.00 x 108 m/s as an approximation for the speed of light, c. Part 2: Different solutions have different spectral (color) properties depending on the wavelengths of light absorbed by the molecules in the solution. For instan ...
... can be used to relate the frequency of light () to its wavelength (). Use 3.00 x 108 m/s as an approximation for the speed of light, c. Part 2: Different solutions have different spectral (color) properties depending on the wavelengths of light absorbed by the molecules in the solution. For instan ...
CHAPTER 7: The Quantum-Mechanical Model of the Atom Energy
... Electromagnetic Radiation Electromagnetic radiation is the emission and transmission of energy in the form of electromagnetic waves. It consists of electric and magnetic field. ...
... Electromagnetic Radiation Electromagnetic radiation is the emission and transmission of energy in the form of electromagnetic waves. It consists of electric and magnetic field. ...
Shear-Plate Collimation Testers Ask About Our Build-to-Print and Custom Capabilities O E M
... screen and a collimation reference line. To collimate an expanded laser beam, the tester is inserted in the beam and the collimator is adjusted until the fringes observed on the screen are parallel to the reference line. All CVI Melles Griot shear-plate modules follow the same sign convention: a con ...
... screen and a collimation reference line. To collimate an expanded laser beam, the tester is inserted in the beam and the collimator is adjusted until the fringes observed on the screen are parallel to the reference line. All CVI Melles Griot shear-plate modules follow the same sign convention: a con ...
Lecture 7 - UIC Department of Chemistry
... A: Heavier the atoms in a diatomic molecule are, smaller the vibrational wavenumber is B: Energy of rotational levels is usually ~ 103 times smaller than that of the corresponding vibrational levels C: Value of rotational constant is dependent on the vibrational energy level D: According to anharmon ...
... A: Heavier the atoms in a diatomic molecule are, smaller the vibrational wavenumber is B: Energy of rotational levels is usually ~ 103 times smaller than that of the corresponding vibrational levels C: Value of rotational constant is dependent on the vibrational energy level D: According to anharmon ...
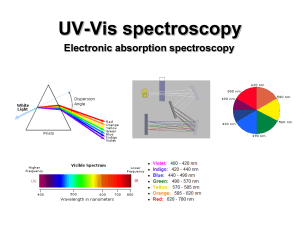
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.