Supplementary Material for
... in which the real part of the spatial field correlation function decays to half its maximum value. The correlation length was determined from measurements made with a collimated incident beam (500-m diameter) with small angular divergence (0.046°). Measurements of for different values of L ...
... in which the real part of the spatial field correlation function decays to half its maximum value. The correlation length was determined from measurements made with a collimated incident beam (500-m diameter) with small angular divergence (0.046°). Measurements of
Chapter1 Fundamental law of geometrical optics 第一章 几何光学的
... ﹡ The fine structure and distribution of light with an image are determined by wave method . ﹡The study of ray phenomenon is known as geometrical optics. The study of light wave is known as physical optics. Ⅲ. Real and Visual images ﹡Real image :formed outside the system, and can be allowed to fall ...
... ﹡ The fine structure and distribution of light with an image are determined by wave method . ﹡The study of ray phenomenon is known as geometrical optics. The study of light wave is known as physical optics. Ⅲ. Real and Visual images ﹡Real image :formed outside the system, and can be allowed to fall ...
Lecture 5: Spectroscopy and Photochemistry I
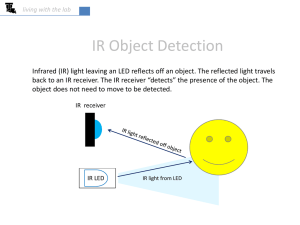
... – Excites vibrational motions in molecules – With a very few exceptions, infrared radiation is not energetic enough to break molecules or initiate photochemical processes ...
... – Excites vibrational motions in molecules – With a very few exceptions, infrared radiation is not energetic enough to break molecules or initiate photochemical processes ...
VCE UNIT 4 SAC
... Information- An experiment was performed that was similar to Young's double slit experiment. One image produced is on the attached sheet. This image resulted from green light of wavelength 520 nm passing through two slits that were separated by 1 mm and then travelled a distance of 80 cm to a screen ...
... Information- An experiment was performed that was similar to Young's double slit experiment. One image produced is on the attached sheet. This image resulted from green light of wavelength 520 nm passing through two slits that were separated by 1 mm and then travelled a distance of 80 cm to a screen ...
Classical Thermodynamics I: Sublimation of Solid Iodine
... about 1 atm to provide pressure broadening of the extremely sharp and intense absorption lines of the rotational fine structure (which can be individually resolved only by special techniques of laser spectroscopy). The reason lies in the logarithmic form of Eq. (34). Within the slit width or resolut ...
... about 1 atm to provide pressure broadening of the extremely sharp and intense absorption lines of the rotational fine structure (which can be individually resolved only by special techniques of laser spectroscopy). The reason lies in the logarithmic form of Eq. (34). Within the slit width or resolut ...
Which frequency of light has the most energy
... Refer to the Bohr model of the hydrogen atom in the chemistry state reference tables Which electron transition would result in emission of ultraviolet radiation? A. Level 4 to level 1 C. Level 5 to level 2 B. Level 4 to level 3 D. Level 3 to level 2 ...
... Refer to the Bohr model of the hydrogen atom in the chemistry state reference tables Which electron transition would result in emission of ultraviolet radiation? A. Level 4 to level 1 C. Level 5 to level 2 B. Level 4 to level 3 D. Level 3 to level 2 ...
Chapter 27
... • Davisson and Germer scattered low-energy electrons from a nickel target and followed this with extensive diffraction measurements from various materials • The wavelength of the electrons calculated from the diffraction data agreed with the expected de Broglie wavelength • This confirmed the wave n ...
... • Davisson and Germer scattered low-energy electrons from a nickel target and followed this with extensive diffraction measurements from various materials • The wavelength of the electrons calculated from the diffraction data agreed with the expected de Broglie wavelength • This confirmed the wave n ...
Figure 7.18 The 3d orbitals
... Figure 7.8 Three series of spectral lines of atomic hydrogen. Balmer is in the visible region and the other series, which have names also, are in uv or ir area of E-M radiation. The Bohr Model of Hydrogen atom 1. H atoms have only certain allowable energy levels called stationary states. 2. At ...
... Figure 7.8 Three series of spectral lines of atomic hydrogen. Balmer is in the visible region and the other series, which have names also, are in uv or ir area of E-M radiation. The Bohr Model of Hydrogen atom 1. H atoms have only certain allowable energy levels called stationary states. 2. At ...
Lecture 10 Activity of chemical components
... where Z is the charge on the ion and I is called as net ionic strength, ci is the concentration of the i th ion. The factor –0.0509 depends on the solvent dielectric constant and temperature. Thus we can see that Debye model predicts a reduction in activity coefficients. (1) Higher the charge, lower ...
... where Z is the charge on the ion and I is called as net ionic strength, ci is the concentration of the i th ion. The factor –0.0509 depends on the solvent dielectric constant and temperature. Thus we can see that Debye model predicts a reduction in activity coefficients. (1) Higher the charge, lower ...
Opt001
... Prism spectrometers are used to measure the wavelengths of light emitted by a sample. The key to its operation is a glass prism, which disperses light into a spectrum. Experiment 1 develops your understanding of how the prism spectrometer works, as well as the skills necessary to using it - adjustme ...
... Prism spectrometers are used to measure the wavelengths of light emitted by a sample. The key to its operation is a glass prism, which disperses light into a spectrum. Experiment 1 develops your understanding of how the prism spectrometer works, as well as the skills necessary to using it - adjustme ...
Solution
... 40) Draw the Lewis structure for sulfate. How many equivalent resonance structures can be drawn? A) 6 B) 2 C) 4 D) 3 E) 8 41) What is the maximum number of f orbitals that are possible? A) 1 B) 3 C) 7 D) 5 E) 9 42) Electromagnetic radiation with a wavelength of 531 nm appears as green light to the h ...
... 40) Draw the Lewis structure for sulfate. How many equivalent resonance structures can be drawn? A) 6 B) 2 C) 4 D) 3 E) 8 41) What is the maximum number of f orbitals that are possible? A) 1 B) 3 C) 7 D) 5 E) 9 42) Electromagnetic radiation with a wavelength of 531 nm appears as green light to the h ...
Light in Modern Physics - Physics | Oregon State University
... the interference pattern of Young's fringes. For the whole interfer ence pattern to be visible many pho tons must contribute to it, with most of the photons landing on the bright places and none at the dark places (Fig. 15.3). However, the same interference pattern results if the light is so faint ...
... the interference pattern of Young's fringes. For the whole interfer ence pattern to be visible many pho tons must contribute to it, with most of the photons landing on the bright places and none at the dark places (Fig. 15.3). However, the same interference pattern results if the light is so faint ...
Chapter 4 Many properties of light can be understood using a wave
... point (crest) on a wave and the rest position; also, the distance between the lowest point (trough) and the ...
... point (crest) on a wave and the rest position; also, the distance between the lowest point (trough) and the ...
Light - FT HELP
... shows how a magnifying glass bends light rays to make things look bigger than they are. Many optical devices use the same basic idea of bending the light to fool your eye and brain so light LOOKS like it came from a different (usually larger or closer) object. OK, now I will tell you something about ...
... shows how a magnifying glass bends light rays to make things look bigger than they are. Many optical devices use the same basic idea of bending the light to fool your eye and brain so light LOOKS like it came from a different (usually larger or closer) object. OK, now I will tell you something about ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.