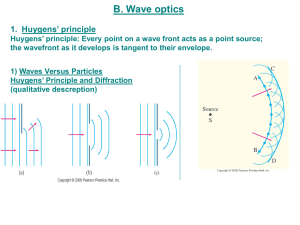
Wave Particle Duality - waiukucollegescience
... ultra-violet radiation of wavelength 2.0 x 10-7m. (5) In an experiment on the photoelectric effect using radiation of wavelength 4.00 x 10-7m the maximum electron energy observed was 1.40 x 10-19 J. With a wavelength 3.00 x 10-7m the maximum energy was 3.06 x 10-19J. Derive a value for Planck's cons ...
... ultra-violet radiation of wavelength 2.0 x 10-7m. (5) In an experiment on the photoelectric effect using radiation of wavelength 4.00 x 10-7m the maximum electron energy observed was 1.40 x 10-19 J. With a wavelength 3.00 x 10-7m the maximum energy was 3.06 x 10-19J. Derive a value for Planck's cons ...
SPECTRAL ANALYSIS
... used in the photoelectric effect experiment. In that experiment, we want to filter out all but the green line of mercury, so we are sure to see the voltage created by light of this one wavelength. In this experiment we will determine how well the filter achieves this. Filter the mercury light throug ...
... used in the photoelectric effect experiment. In that experiment, we want to filter out all but the green line of mercury, so we are sure to see the voltage created by light of this one wavelength. In this experiment we will determine how well the filter achieves this. Filter the mercury light throug ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
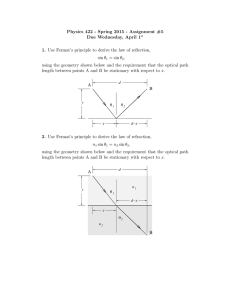
... (b) How would you use it to determine the wavelength of a monochromatic light and refractive index of a thin transparent sheet? 18. (a) Discuss Fraunhofer diffraction pattern of a straight edge. ...
... (b) How would you use it to determine the wavelength of a monochromatic light and refractive index of a thin transparent sheet? 18. (a) Discuss Fraunhofer diffraction pattern of a straight edge. ...
Arrangement of Electrons in Atoms I. The Development of a New
... _____________________________ only in small, specific amounts called ___________________. A quantum is the minimum amount of energy that can be ______________________ or ________________________ by an atom. 2. A direct proportion exists between the energy of a quantum and the frequency of the emitte ...
... _____________________________ only in small, specific amounts called ___________________. A quantum is the minimum amount of energy that can be ______________________ or ________________________ by an atom. 2. A direct proportion exists between the energy of a quantum and the frequency of the emitte ...
revised preliminary introduction of spectroscopy
... Ultraviolet-Visible (UV-VIS) spectroscopy is useful to characterize the absorption, transmission, and reflectivity of a variety of technologically important materials, such as pigments, coatings etc. The UV-VIS spectroscopy is usually applied to molecules or inorganic complexes in solution, for exam ...
... Ultraviolet-Visible (UV-VIS) spectroscopy is useful to characterize the absorption, transmission, and reflectivity of a variety of technologically important materials, such as pigments, coatings etc. The UV-VIS spectroscopy is usually applied to molecules or inorganic complexes in solution, for exam ...
Combining and Choosing Analytical Techniques
... The sample as a gas enters the evacuated tube. Positive ions are formed in the ionisation chamber when an electron beam dislodges electrons from the sample atoms The positive ions are accelerated by an electric field The ions enter a magnetic field perpendicular to their path. This causes the ions t ...
... The sample as a gas enters the evacuated tube. Positive ions are formed in the ionisation chamber when an electron beam dislodges electrons from the sample atoms The positive ions are accelerated by an electric field The ions enter a magnetic field perpendicular to their path. This causes the ions t ...
Spectral lines, wavelength of light, Rydberg constant
... in the opposite direction. How do the two first order maxima compare? Can you find the second order maximum? How does it compare with the first order? Calculate the wavelength range of visible light for your measurements. 3) Replace the incandescent source with either a helium or mercury source. Rec ...
... in the opposite direction. How do the two first order maxima compare? Can you find the second order maximum? How does it compare with the first order? Calculate the wavelength range of visible light for your measurements. 3) Replace the incandescent source with either a helium or mercury source. Rec ...
Spectroscopy - Birmingham City Schools
... Maxwell and Boltzman (we’ll hear more about him in future units…) showed that this model explained everything we knew about heat and Temp. o Absorption of EM radiation increases temperature o Reflected EM radiation shows color (if in visible region) But… What controls whether radiation is absorbed ...
... Maxwell and Boltzman (we’ll hear more about him in future units…) showed that this model explained everything we knew about heat and Temp. o Absorption of EM radiation increases temperature o Reflected EM radiation shows color (if in visible region) But… What controls whether radiation is absorbed ...
Lecture 24: Quantum mechanics
... wavelength it would look like the series of spectra shown to the left. Note it has a distinct maximum characteristic of its temperature. The position of the maximum shifts towards a lower wavelength (Blue shift) as the body temperature is increased. In the late nineteenth century, Raleigh showed tha ...
... wavelength it would look like the series of spectra shown to the left. Note it has a distinct maximum characteristic of its temperature. The position of the maximum shifts towards a lower wavelength (Blue shift) as the body temperature is increased. In the late nineteenth century, Raleigh showed tha ...
Daedalon EO-85 Computerized Spectrophotometer
... appears to be a single color. The goal of this part is to measure the spectrum and determine the width of the wavelength band that is emitted in each case. 2. Measure the emission spectrum of the green, red and blue light emitting diodes in that order. The graphs will then be the same color as the L ...
... appears to be a single color. The goal of this part is to measure the spectrum and determine the width of the wavelength band that is emitted in each case. 2. Measure the emission spectrum of the green, red and blue light emitting diodes in that order. The graphs will then be the same color as the L ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.