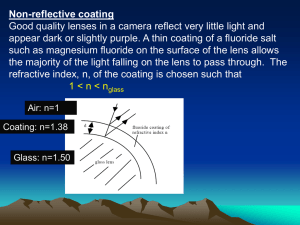
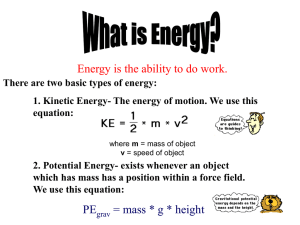
Light
... and destructive interference. Or light may act similar to a bullet and actually cause matter be relocated. This represents the particle like property of light. The latter property can be explained by the Photoelectric effect. ...
... and destructive interference. Or light may act similar to a bullet and actually cause matter be relocated. This represents the particle like property of light. The latter property can be explained by the Photoelectric effect. ...
CHAPTER 3: Light and Telescopes
... There are a wide range of wavelengths of electromagnetic waves plotted on the electromagnetic spectrum. We classify these waves by their source, use, or interactions with other matter. Only a very small range of wavelengths, 400nm to 700nm, are visible to humans. (Since these wavelengths are small w ...
... There are a wide range of wavelengths of electromagnetic waves plotted on the electromagnetic spectrum. We classify these waves by their source, use, or interactions with other matter. Only a very small range of wavelengths, 400nm to 700nm, are visible to humans. (Since these wavelengths are small w ...
slides introducing IR/Raman of proteins
... occur but are weak. These are termed overtones (D vi = ± 2,± 3, . .) or combination bands (D vi = ± 1, D vj = ± 1, . .). ...
... occur but are weak. These are termed overtones (D vi = ± 2,± 3, . .) or combination bands (D vi = ± 1, D vj = ± 1, . .). ...
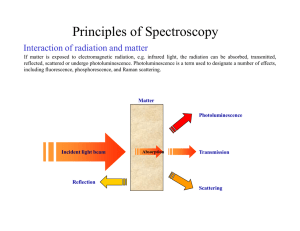
Principles of Spectroscopy
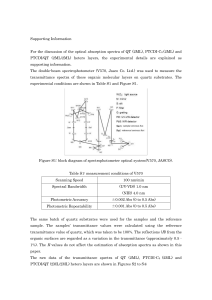
... Limitations of the Beer-Lambert law The linearity of the Beer-Lambert law is limited by chemical and instrumental factors. Causes of nonlinearity include: • deviations in absorptivity coefficients at high concentrations (>0.01M) due to electrostatic interactions between molecules in close proximity ...
... Limitations of the Beer-Lambert law The linearity of the Beer-Lambert law is limited by chemical and instrumental factors. Causes of nonlinearity include: • deviations in absorptivity coefficients at high concentrations (>0.01M) due to electrostatic interactions between molecules in close proximity ...
Physics 234 Exam # 2 Review
... 9. (15 pts) The first-order reflection from the reflection planes shown occurs when an xray beam of wavelength 0.260 nm makes an angle of 63.8° with the top face of the crystal. What is the unit cell size a0? ...
... 9. (15 pts) The first-order reflection from the reflection planes shown occurs when an xray beam of wavelength 0.260 nm makes an angle of 63.8° with the top face of the crystal. What is the unit cell size a0? ...
IODINE, IODIDE, TRI-IODIDE EQUILIBRIUM (Rev`d 3/25
... where aI3- represents the activity of tri-iodide which is approximately equivalent to the stoichiometric concentration of the tri-iodide ion, [I3-] at dilute concentrations. The variables for iodine and iodide are defined in an analogous manner. Equation 3 is the Beer's law relationship for absorban ...
... where aI3- represents the activity of tri-iodide which is approximately equivalent to the stoichiometric concentration of the tri-iodide ion, [I3-] at dilute concentrations. The variables for iodine and iodide are defined in an analogous manner. Equation 3 is the Beer's law relationship for absorban ...
Photoelectric Effect When light shines on a metal surface, electrons are emitted
... Photoelectric Effect When light shines on a metal surface, electrons are emitted from the surface. ...
... Photoelectric Effect When light shines on a metal surface, electrons are emitted from the surface. ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.