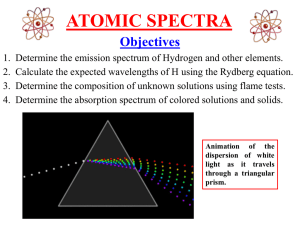
Atomic_spectra
... Flame test knowns – in hoods Flame test unknowns – vials at bench by blackboard 12M HCl for cleaning loops – in “Beilstein” hood Gas discharge tubes (for viewing by STAR spectroscope) – in 201 Computerized spectrophotometer – 1 setup in 201 Cobalt Chloride solution – at bench by blackboard Colored s ...
... Flame test knowns – in hoods Flame test unknowns – vials at bench by blackboard 12M HCl for cleaning loops – in “Beilstein” hood Gas discharge tubes (for viewing by STAR spectroscope) – in 201 Computerized spectrophotometer – 1 setup in 201 Cobalt Chloride solution – at bench by blackboard Colored s ...
Module 1 - Identifying Metals Using Atomic Emission
... Hollow Cathode Lamps—the most common radiation source in AAS. Inside the sealed lamp, filled with argon or neon gas at low pressure, is a cylindrical metal cathode containing the element of interest and an anode. A high voltage is applied across the anode and cathode, resulting in an ionization of t ...
... Hollow Cathode Lamps—the most common radiation source in AAS. Inside the sealed lamp, filled with argon or neon gas at low pressure, is a cylindrical metal cathode containing the element of interest and an anode. A high voltage is applied across the anode and cathode, resulting in an ionization of t ...
Photosynthesis in plants requires sunlight in addition
... A typical absorption spectrum of green leaved plants is shown below. It shows a prominent broad dip in the green region and high absorption in wavelength regions above 600 nm and below 500 nm. ...
... A typical absorption spectrum of green leaved plants is shown below. It shows a prominent broad dip in the green region and high absorption in wavelength regions above 600 nm and below 500 nm. ...
Measurement of Optical Characteristic of Plastic by UH4150
... new model developed focusing on the photometric accuracy for solids such as optical materials. UH4150 is the spectrophotometer which inherited the optical system of U-4100 having an established reputation for its parallel beam and low polarization property that are suitable for the optical material ...
... new model developed focusing on the photometric accuracy for solids such as optical materials. UH4150 is the spectrophotometer which inherited the optical system of U-4100 having an established reputation for its parallel beam and low polarization property that are suitable for the optical material ...
what is energy?
... SPACE AS A PHOTON (PACKET OF ENERGY). • IT IS PART OF THE ELECTROMAGNETIC SPECTRUM. • LIGHT CAN BE REFLECTED, REFRACTED AND DIFFRACTED ...
... SPACE AS A PHOTON (PACKET OF ENERGY). • IT IS PART OF THE ELECTROMAGNETIC SPECTRUM. • LIGHT CAN BE REFLECTED, REFRACTED AND DIFFRACTED ...
Introduction to Spectroscopic Methods ver.2
... A = log Psolvent/Psolution x log Po/P In order to make manual photometers and spectrophotometers, which are often equipped with a display that has a linear scale extending from 0 to a 100%, operate in such a way that their readings are in percent transmittance, two preliminary adjustments are requi ...
... A = log Psolvent/Psolution x log Po/P In order to make manual photometers and spectrophotometers, which are often equipped with a display that has a linear scale extending from 0 to a 100%, operate in such a way that their readings are in percent transmittance, two preliminary adjustments are requi ...
Using a Spectrophotometer
... The perception of color, as just described is qualitative. It indicates what is happening but says nothing about the extent to which the event is taking place. The eye is not a quantitative instrument. However, there are instruments, called spectrophotometers, which electronically quantify the amoun ...
... The perception of color, as just described is qualitative. It indicates what is happening but says nothing about the extent to which the event is taking place. The eye is not a quantitative instrument. However, there are instruments, called spectrophotometers, which electronically quantify the amoun ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.