Why The Sky Is Blue
... firstly, sunlight is not uniformly intense at all wavelengths (otherwise it would be pure white before entering the atmosphere). It has a peak intensity somewhere in the green part of the visible spectrum, so the entering intensity of violet light is considerably less than that of blue. The other re ...
... firstly, sunlight is not uniformly intense at all wavelengths (otherwise it would be pure white before entering the atmosphere). It has a peak intensity somewhere in the green part of the visible spectrum, so the entering intensity of violet light is considerably less than that of blue. The other re ...
Light Bulbs
... Electricity excites gas molecules in light bulb Most energy is converted into visible light instead of heat. Mercury is highly toxic chemical ...
... Electricity excites gas molecules in light bulb Most energy is converted into visible light instead of heat. Mercury is highly toxic chemical ...
Beam Splitters A beam splitter is a device that`s used to divide an
... A beam splitter is a device that’s used to divide an optical beam into two or more components according to given criteria. Beam splitters can be grouped into the following four classifications. These classes include those beam splitters that divide the beam size according to beam intensity, waveleng ...
... A beam splitter is a device that’s used to divide an optical beam into two or more components according to given criteria. Beam splitters can be grouped into the following four classifications. These classes include those beam splitters that divide the beam size according to beam intensity, waveleng ...
Appendix I.
... This X-ray technique utilizes different material characteristics for identification purposes. Rather than establishing which elements are present, the XRD exploits the diffraction of an incident X-ray with a substance whose structure is crystalline and is therefore composed of repeating units. When ...
... This X-ray technique utilizes different material characteristics for identification purposes. Rather than establishing which elements are present, the XRD exploits the diffraction of an incident X-ray with a substance whose structure is crystalline and is therefore composed of repeating units. When ...
lecture #7 ppt
... February 13 | 4-5 p.m. | Pitzer Auditorium, 120 Latimer Hall Professor Thomas F. Kuech, Dept. of Chemical & Biological Engineering, University of Wisconsin - Madison Using Supported Lipid Bilayers as a Separation Matrix February 15 | 4-5 p.m. | 775A Tan Hall Professor Paul Cremer, Dept. of Chemistry ...
... February 13 | 4-5 p.m. | Pitzer Auditorium, 120 Latimer Hall Professor Thomas F. Kuech, Dept. of Chemical & Biological Engineering, University of Wisconsin - Madison Using Supported Lipid Bilayers as a Separation Matrix February 15 | 4-5 p.m. | 775A Tan Hall Professor Paul Cremer, Dept. of Chemistry ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 4. In Newton’s rings experiment, the diameter of the 8th ring changes from 1.4cm to 1.27cm when a liquid is introduced between the lens and the plate. Calculate the refractive index of the liquid. 5. Define resolving power of an optical instrument. 6. Compare a zone plate and a convex lens. 7. What ...
... 4. In Newton’s rings experiment, the diameter of the 8th ring changes from 1.4cm to 1.27cm when a liquid is introduced between the lens and the plate. Calculate the refractive index of the liquid. 5. Define resolving power of an optical instrument. 6. Compare a zone plate and a convex lens. 7. What ...
HW8 not graded v3 - Department of Physics | Oregon State
... An electron orbiting a proton has an ill-defined de Broglie wavelength (because there is uncertainty in the electron’s momentum). However, we can say that the likely de Broglie wavelengths of such an electron are of order of magnitude 3 x 10-10 m. ...
... An electron orbiting a proton has an ill-defined de Broglie wavelength (because there is uncertainty in the electron’s momentum). However, we can say that the likely de Broglie wavelengths of such an electron are of order of magnitude 3 x 10-10 m. ...
PHYS 203 General Physics
... The final exam is Monday, June 7, at 1:00 pm. The exam will be comprehensive. It will be closed book. All necessary equations will be provided, but you may bring a 3 × 5 card with equations written on it if you wish. You are also responsible for George Gamow’s book, Chapters 1 through 6. Look over t ...
... The final exam is Monday, June 7, at 1:00 pm. The exam will be comprehensive. It will be closed book. All necessary equations will be provided, but you may bring a 3 × 5 card with equations written on it if you wish. You are also responsible for George Gamow’s book, Chapters 1 through 6. Look over t ...
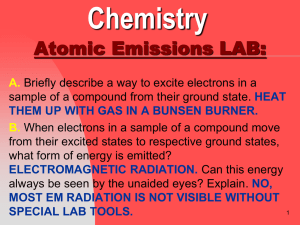
atomic emission spectrum
... this frequency into wavelength (nm). Does this frequency fall in the visible region? ...
... this frequency into wavelength (nm). Does this frequency fall in the visible region? ...
OOSpecActivities
... surprises. Only red, green, and blue pixels can be seen on a monitor. The computer adjusts the relative brightness of each color to produce many possible combinations. This works because the human eye can only detect these 3 colors. Other colors stimulate the 3 color receptors (cones) to different d ...
... surprises. Only red, green, and blue pixels can be seen on a monitor. The computer adjusts the relative brightness of each color to produce many possible combinations. This works because the human eye can only detect these 3 colors. Other colors stimulate the 3 color receptors (cones) to different d ...
Teaching program
... Discuss thin film interference and practical situations such as colours of oil films, colours of butterfly wings Discuss how to obtain path difference between the two layers of the film Extend this concept to X ray reflection from parallel plans in a crystal and to Bragg’s Law ...
... Discuss thin film interference and practical situations such as colours of oil films, colours of butterfly wings Discuss how to obtain path difference between the two layers of the film Extend this concept to X ray reflection from parallel plans in a crystal and to Bragg’s Law ...
Introduction to Spectroscopy
... Interactions with matter • Ionizing – enough energy to liberate e• Non-ionizing – in general: reflection, transmission or absorption – Absorbed radiation may be re-radiated (scattered) at the original frequency (Rayleigh scattering) or at a different frequency (Raman, Brillouin, fluorescence, etc.) ...
... Interactions with matter • Ionizing – enough energy to liberate e• Non-ionizing – in general: reflection, transmission or absorption – Absorbed radiation may be re-radiated (scattered) at the original frequency (Rayleigh scattering) or at a different frequency (Raman, Brillouin, fluorescence, etc.) ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.