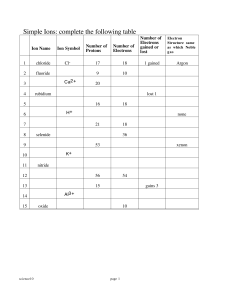
Periodicity of Elements
... The atomic number tells how many. protons the atom of that element contains in the nucleus. Use the periodic table in your textbook, or another periodic table, to look up the a t ~ m i cnumbers. It will be the whole number just above the element symbol. To find the answer to the question, use only t ...
... The atomic number tells how many. protons the atom of that element contains in the nucleus. Use the periodic table in your textbook, or another periodic table, to look up the a t ~ m i cnumbers. It will be the whole number just above the element symbol. To find the answer to the question, use only t ...
The Periodic Table - Anderson High School
... number of valence electrons. • They will form the same kinds of ions. ...
... number of valence electrons. • They will form the same kinds of ions. ...
The Advanced Placement Examination in Chemistry Part I – Multiple
... (d) Compound Z contains carbon, hydrogen, and element Q. When 1.00 gram of compound Z is oxidized and all of the carbon and hydrogen are converted to oxides, 1.37 grams of CO2 and 0.281 gram of water are produced. Determine the most probable molecular formula of compound Z. ...
... (d) Compound Z contains carbon, hydrogen, and element Q. When 1.00 gram of compound Z is oxidized and all of the carbon and hydrogen are converted to oxides, 1.37 grams of CO2 and 0.281 gram of water are produced. Determine the most probable molecular formula of compound Z. ...
Review guide for Chemistry`s First Semester Exam Unit 1 Thinking
... Unit 4: Copper lab, Writing formulas, Naming compounds Define Reactant and products, precipitate, exothermic and endothermic reactions and signs of each Write the definition of the following terms: 1. Reactants and products 2. Precipitate 3. Exothermic reaction and endothermic reaction Define the s ...
... Unit 4: Copper lab, Writing formulas, Naming compounds Define Reactant and products, precipitate, exothermic and endothermic reactions and signs of each Write the definition of the following terms: 1. Reactants and products 2. Precipitate 3. Exothermic reaction and endothermic reaction Define the s ...
GCE Chemistry Question Paper Unit 04 - Kinetics, Equilibria
... Calculate the amounts, in moles, of hydrogen and of iodine in the equilibrium mixture. Moles of hydrogen ............................................................................................................. Moles of iodine ..................................................................... ...
... Calculate the amounts, in moles, of hydrogen and of iodine in the equilibrium mixture. Moles of hydrogen ............................................................................................................. Moles of iodine ..................................................................... ...
Periodic Table Notes
... TEKS 8.5C interpret the arrangement of the Periodic Table, including groups and periods, to explain how properties are used to classify elements TEKS 8.5B identify that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity ...
... TEKS 8.5C interpret the arrangement of the Periodic Table, including groups and periods, to explain how properties are used to classify elements TEKS 8.5B identify that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity ...
Chemistry II Exams and Keys 2014 Season
... 22. Green light has sufficient energy to break the bond between two chlorine atoms. Yellow light cannot break the bond between two chlorine atoms. Which of the following statements are correct? Use these values for the average bond strengths: Br-Br (193 kJ/mol) and Cl-Cl (233 kJ/mol). I. Blue light ...
... 22. Green light has sufficient energy to break the bond between two chlorine atoms. Yellow light cannot break the bond between two chlorine atoms. Which of the following statements are correct? Use these values for the average bond strengths: Br-Br (193 kJ/mol) and Cl-Cl (233 kJ/mol). I. Blue light ...
Periodicity Chemistry Worksheet
... 10. The distance from the nucleus to the outer most electron is known as _Atomic Radius___. 11. The _noble gases_ do not have measured electronegativites since they do not commonly ...
... 10. The distance from the nucleus to the outer most electron is known as _Atomic Radius___. 11. The _noble gases_ do not have measured electronegativites since they do not commonly ...
The History of the Modern Periodic Table
... copy of that, which had similar elements grouped in vertical columns, unlike his first table, which grouped them horizontally. These historic documents still exist. That Mendeleev realized that he had discovered, rather than designed, the periodic table is shown by his attitude towards it. ...
... copy of that, which had similar elements grouped in vertical columns, unlike his first table, which grouped them horizontally. These historic documents still exist. That Mendeleev realized that he had discovered, rather than designed, the periodic table is shown by his attitude towards it. ...
02 The structure of the periodic table II
... 1) He insisted that chemically similar elements be listed in the same columns (groups) on the table ...
... 1) He insisted that chemically similar elements be listed in the same columns (groups) on the table ...
Chapter Test B
... electrons from another atom in the compound is called _______________________. 17. The energy required to remove one electron from an atom is called its _______________________. 18. The valence electron configuration for the Group 16 element in Period 3 is _______________________. 19. One-half the d ...
... electrons from another atom in the compound is called _______________________. 17. The energy required to remove one electron from an atom is called its _______________________. 18. The valence electron configuration for the Group 16 element in Period 3 is _______________________. 19. One-half the d ...
Structures and Bonding
... 2) Recycled metals only need about 1/10th of the energy to produce compared to producing new metals 3) Recycling paper reduces the amount of water and energy needed to produce it 4) Recycled glass only needs 80% of the energy to produce compared to producing new glass 5) Recycling saves on raw mater ...
... 2) Recycled metals only need about 1/10th of the energy to produce compared to producing new metals 3) Recycling paper reduces the amount of water and energy needed to produce it 4) Recycled glass only needs 80% of the energy to produce compared to producing new glass 5) Recycling saves on raw mater ...
Lesson 7.8 Basic Properties of the Main Group Elements Suggested
... chlorine, Cl2, a pale greenish-yellow gas; bromine, Br2 , a reddish-brown liquid; and iodine, I2, a bluish-black solid that gives off violet vapor. Little is known about astatine, At, because it is a synthetic elements and is radioactive. It is expected be more metallic than iodine and is perhaps a ...
... chlorine, Cl2, a pale greenish-yellow gas; bromine, Br2 , a reddish-brown liquid; and iodine, I2, a bluish-black solid that gives off violet vapor. Little is known about astatine, At, because it is a synthetic elements and is radioactive. It is expected be more metallic than iodine and is perhaps a ...
10th CBSE {SA - 1} Revision Pack Booklet - 3
... It is prevented by using antioxidants or cutting the supply of oxygen. Chips packets are flushed with nitrogen gas to shield the chips from oxygen supply. Nitrogen does not cause rancidity of food. This helps in preserving the taste and smell of food. Manufacturers and shopkeepers are helped as shel ...
... It is prevented by using antioxidants or cutting the supply of oxygen. Chips packets are flushed with nitrogen gas to shield the chips from oxygen supply. Nitrogen does not cause rancidity of food. This helps in preserving the taste and smell of food. Manufacturers and shopkeepers are helped as shel ...
Chemistry Online Textbook
... total. Only 255 of these naturally occurring isotopes are stable in the sense of never having been observed to decay as of the present time. All the known stable isotopes occur naturally on Earth; the other naturally occurring-isotopes are radioactive but occur on Earth due to their relatively long ...
... total. Only 255 of these naturally occurring isotopes are stable in the sense of never having been observed to decay as of the present time. All the known stable isotopes occur naturally on Earth; the other naturally occurring-isotopes are radioactive but occur on Earth due to their relatively long ...
Chapter 7 Periodic Properties of Elements - GCG-42
... •When chlorine forms an anion by adding an electron, it has the same electron configuration as argon the noble gas, [Ar]. The radius of a chloride ion is larger than the chlorine atom. It is also larger than the argon ...
... •When chlorine forms an anion by adding an electron, it has the same electron configuration as argon the noble gas, [Ar]. The radius of a chloride ion is larger than the chlorine atom. It is also larger than the argon ...
workbook Chem (WP)
... 1. Identify and define the types of pure substances. 2. Identify and define the two main types of mixtures. Chemical vs Physical Properties 1. Identify the difference between a chemical and a physical change. ...
... 1. Identify and define the types of pure substances. 2. Identify and define the two main types of mixtures. Chemical vs Physical Properties 1. Identify the difference between a chemical and a physical change. ...
Lesson 1 - Scientist in Residence
... This lab introduces the periodic table, the structure of the atom, and how the positions of the elements in the periodic table relate to conduction and insulation. An element is a piece of matter in its simplest form. All matter (solid, liquid, gas) is made of atoms. Atoms join together to make mole ...
... This lab introduces the periodic table, the structure of the atom, and how the positions of the elements in the periodic table relate to conduction and insulation. An element is a piece of matter in its simplest form. All matter (solid, liquid, gas) is made of atoms. Atoms join together to make mole ...
Periodic Table
... Known properties were: melting point, density, color, atomic mass, # of chemical bonds an element can form. Atomic mass is the average mass of one atom of that element. ...
... Known properties were: melting point, density, color, atomic mass, # of chemical bonds an element can form. Atomic mass is the average mass of one atom of that element. ...
Periodicity - ilc.edu.hk
... are great ∵ extent of bond breaking : boiling >> melting Particles are completely separated on boiling For Gp4A elements, the differences between m.p. and b.p. are relatively small ∵ extent of bond breaking : boiling melting ...
... are great ∵ extent of bond breaking : boiling >> melting Particles are completely separated on boiling For Gp4A elements, the differences between m.p. and b.p. are relatively small ∵ extent of bond breaking : boiling melting ...
HS standard 4 2017
... You can predict the properties of elements based on their location in the periodic table. As you move across the periodic table elements change from metals to nonmetals. ...
... You can predict the properties of elements based on their location in the periodic table. As you move across the periodic table elements change from metals to nonmetals. ...
Aqueous Reactions
... dissociate into separate ions in water. However, not all electrolytes behave the same way. Some are strong electrolytes, and dissociate completely, so no ions are left bonded together. Others are weak electrolytes- they only partly dissociate, and many of their ions are still bonded to each other. O ...
... dissociate into separate ions in water. However, not all electrolytes behave the same way. Some are strong electrolytes, and dissociate completely, so no ions are left bonded together. Others are weak electrolytes- they only partly dissociate, and many of their ions are still bonded to each other. O ...