Systems and Surroundings
... of work. We shall look at two ways, each of which has its own formula for calculating the work. 1) Constant external pressure, Pex This is path B in the diagram below. In a first step, the volume is held constant by keeping the piston in place while adjusting the Pex from 3.00 atm to 1.2 atm. Then i ...
... of work. We shall look at two ways, each of which has its own formula for calculating the work. 1) Constant external pressure, Pex This is path B in the diagram below. In a first step, the volume is held constant by keeping the piston in place while adjusting the Pex from 3.00 atm to 1.2 atm. Then i ...
Course Pack3 Phase Diagrams
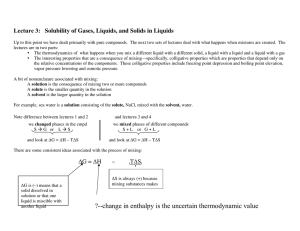
... ∆Hsoln is (+) for NaCl in H2O ∆Hsoln is (–) for Na2SO4 in H2O ∆Hsoln is (–) for O2 in H2O Consider the case that ∆Hmix is negative: since ∆Smix is positive then ∆Gsoln will have to be negative and the reaction happens. Now consider the case that ∆Hmix is positive: in this case the spontaneity of the ...
... ∆Hsoln is (+) for NaCl in H2O ∆Hsoln is (–) for Na2SO4 in H2O ∆Hsoln is (–) for O2 in H2O Consider the case that ∆Hmix is negative: since ∆Smix is positive then ∆Gsoln will have to be negative and the reaction happens. Now consider the case that ∆Hmix is positive: in this case the spontaneity of the ...
Chapter 19 The First Law of Thermodynamics
... To represent heat transfer and work done in a thermodynamic process and to calculate work To relate heat transfer, work done, and internal energy change using the first law of thermodynamics To distinguish between adiabatic, isochoric, isobaric, and isothermal processes ...
... To represent heat transfer and work done in a thermodynamic process and to calculate work To relate heat transfer, work done, and internal energy change using the first law of thermodynamics To distinguish between adiabatic, isochoric, isobaric, and isothermal processes ...
LECTURE 7 General Relations for a Homogeneous Substance For
... less kinetic energy in ice than in water. Also the water molecules are, on average, farther from each other in ice than in water, so their potential energy of interaction is less in ice than in water. But ice only exists at low temperatures. Why is that? Because at high temperatures the free energy ...
... less kinetic energy in ice than in water. Also the water molecules are, on average, farther from each other in ice than in water, so their potential energy of interaction is less in ice than in water. But ice only exists at low temperatures. Why is that? Because at high temperatures the free energy ...
Chapter 15
... Metabolism--the many energy transforming processes that occur within an organism U = Q - W our bodies does work on environment and loses heat so to maintain our internal energy we must increase U by eating metabolic rate--rate at which U is transformed inside our body (kcal/h or watts) ...
... Metabolism--the many energy transforming processes that occur within an organism U = Q - W our bodies does work on environment and loses heat so to maintain our internal energy we must increase U by eating metabolic rate--rate at which U is transformed inside our body (kcal/h or watts) ...
Thermodynamics
... [usually air or water]. Ever heard heat rises? It doesn’t. It only moves from hot to cold! What happens say at a campfire, is that the air next to the flame is heated, expands, becomes less dense and floats up to your outstretched, cold hands that are ABOVE the flame. Ever see the road look wet off ...
... [usually air or water]. Ever heard heat rises? It doesn’t. It only moves from hot to cold! What happens say at a campfire, is that the air next to the flame is heated, expands, becomes less dense and floats up to your outstretched, cold hands that are ABOVE the flame. Ever see the road look wet off ...
Model Question Paper – 1
... volume of 0.22 m3 to a final pressure of 100 kPa in a process described by PV1.2 = constant. a) If expansion is quasi-static, find heat transfer, work transfer and change in internal energy. b) In another process the same system expands according to the same P-v relation as in part (a); and between ...
... volume of 0.22 m3 to a final pressure of 100 kPa in a process described by PV1.2 = constant. a) If expansion is quasi-static, find heat transfer, work transfer and change in internal energy. b) In another process the same system expands according to the same P-v relation as in part (a); and between ...
Entropy Analysis of Pressure Driven Flow in a Curved Duct
... Peristaltic pumping is a kind of fluid transport that occurs when progressive wave of contraction and expansion passed along a conduit containing liquid. The fluid mechanics of this phenomenon has been studied by several researchers because of its application in biology and industry. Sato et al. [1] ...
... Peristaltic pumping is a kind of fluid transport that occurs when progressive wave of contraction and expansion passed along a conduit containing liquid. The fluid mechanics of this phenomenon has been studied by several researchers because of its application in biology and industry. Sato et al. [1] ...
Heat Capacity (C)
... Heat transfer occurs when two objects at a different temperature come in contact. The flow of heat will continue until both objects reach the same temperature, this is known as “thermal equilibrium”. Two types of heat flow can be defined: Endothermic Process: Heat flows from the surroundings to the ...
... Heat transfer occurs when two objects at a different temperature come in contact. The flow of heat will continue until both objects reach the same temperature, this is known as “thermal equilibrium”. Two types of heat flow can be defined: Endothermic Process: Heat flows from the surroundings to the ...
Lecture 4: 09.16.05 Temperature, heat, and entropy
... o� Why would two metals heated to the same temperature have a different ability to melt the wax strip? The answer lies in the equation above relating temperature and heat, which indicates that two metals at the same temperature have not necessarily received the same amount of energysince the amount ...
... o� Why would two metals heated to the same temperature have a different ability to melt the wax strip? The answer lies in the equation above relating temperature and heat, which indicates that two metals at the same temperature have not necessarily received the same amount of energysince the amount ...
Calculations Formulas Definitions
... 6. Faraday’s Laws First Law: The mass of a substance reacting at the electrodes is directly proportional to the quantity of electricity passed through the solution. Second Law: The masses of different substances produced during electrolysis are directly proportional to their equivalent weights; 96, ...
... 6. Faraday’s Laws First Law: The mass of a substance reacting at the electrodes is directly proportional to the quantity of electricity passed through the solution. Second Law: The masses of different substances produced during electrolysis are directly proportional to their equivalent weights; 96, ...
Countercurrent exchange

Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some component, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or even solid powders, or any combination of those. For example, in a distillation column, the vapors bubble up through the downward flowing liquid while exchanging both heat and mass.The maximum amount of heat or mass transfer that can be obtained is higher with countercurrent than co-current (parallel) exchange because countercurrent maintains a slowly declining difference or gradient (usually temperature or concentration difference). In cocurrent exchange the initial gradient is higher but falls off quickly, leading to wasted potential. For example, in the diagram at the right, the fluid being heated (exiting top) has a higher exiting temperature than the cooled fluid (exiting bottom) that was used for heating. With cocurrent or parallel exchange the heated and cooled fluids can only approach one another. The result is that countercurrent exchange can achieve a greater amount of heat or mass transfer than parallel under otherwise similar conditions. See: flow arrangement.Countercurrent exchange when set up in a circuit or loop can be used for building up concentrations, heat, or other properties of flowing liquids. Specifically when set up in a loop with a buffering liquid between the incoming and outgoing fluid running in a circuit, and with active transport pumps on the outgoing fluid's tubes, the system is called a Countercurrent multiplier, enabling a multiplied effect of many small pumps to gradually build up a large concentration in the buffer liquid.Other countercurrent exchange circuits where the incoming and outgoing fluids touch each other are used for retaining a high concentration of a dissolved substance or for retaining heat, or for allowing the external buildup of the heat or concentration at one point in the system.Countercurrent exchange circuits or loops are found extensively in nature, specifically in biologic systems. In vertebrates, they are called a Rete mirabile, originally the name of an organ in fish gills for absorbing oxygen from the water. It is mimicked in industrial systems. Countercurrent exchange is a key concept in chemical engineering thermodynamics and manufacturing processes, for example in extracting sucrose from sugar beet roots.Countercurrent multiplication is a similar but different concept where liquid moves in a loop followed by a long length of movement in opposite directions with an intermediate zone. The tube leading to the loop passively building up a gradient of heat (or cooling) or solvent concentration while the returning tube has a constant small pumping action all along it, so that a gradual intensification of the heat or concentration is created towards the loop. Countercurrent multiplication has been found in the kidneys as well as in many other biological organs.