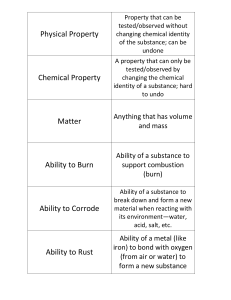
PX121: Thermal Physics Lecture 2
... Leads to “Zeroeth Law of Thermodynamics: “If systems S1 and S2 are separately in thermal equilibrium with system S3 then they are in thermal equilibrium with each other.” Results in the idea that there is something you could measure separately about S1 and S2 which would be the same. This “something ...
... Leads to “Zeroeth Law of Thermodynamics: “If systems S1 and S2 are separately in thermal equilibrium with system S3 then they are in thermal equilibrium with each other.” Results in the idea that there is something you could measure separately about S1 and S2 which would be the same. This “something ...
If we regard the alveoli as spherical bubles, then - Lectures For UG-5
... to inadequate formation of surfactant resulting in elevated alveolar surface tension, increased work of breathing and inadequate exchange of gases. ...
... to inadequate formation of surfactant resulting in elevated alveolar surface tension, increased work of breathing and inadequate exchange of gases. ...
Thermochemistry
... Specific heat is represented by C. The units of specific heat are J/goC. Water has a higher specific heat than most substances. ...
... Specific heat is represented by C. The units of specific heat are J/goC. Water has a higher specific heat than most substances. ...
principles of reactivity: energy and chemical reactions
... The total thermal energy in an object is the sum of its individual energies of all of the molecules. For any given substance, its thermal energy depends not only on its composition, but also on the amount of substance. ...
... The total thermal energy in an object is the sum of its individual energies of all of the molecules. For any given substance, its thermal energy depends not only on its composition, but also on the amount of substance. ...
Thermoregulation
... in response to rising temperature, the body increases blood flow to the skin is controlled by the autonomic nervous system results in an increase in blood flow to the skin, allowing heat loss via radiation, conduction, convection and evaporation prompts cooling of the blood that is flowing t ...
... in response to rising temperature, the body increases blood flow to the skin is controlled by the autonomic nervous system results in an increase in blood flow to the skin, allowing heat loss via radiation, conduction, convection and evaporation prompts cooling of the blood that is flowing t ...
Thermodynamics
... Thermodynamics is the study that concerns with the ways energy is stored within a body and how energy transformations, which involve heat and work, may take place. ...
... Thermodynamics is the study that concerns with the ways energy is stored within a body and how energy transformations, which involve heat and work, may take place. ...
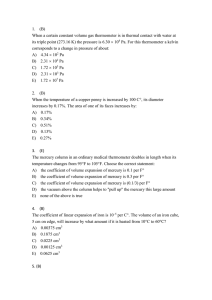
12.1 Thermodynamic Systems, States, and Processes 12.3
... highest possible value, (b) lowest possible value, (c) average value, (d) none of the preceding. (a) It has been proposed that temperature differences in the ocean could be used to run a heat engine to generate electricity. In tropical regions, the water temperature is about 25°C at the surface an ...
... highest possible value, (b) lowest possible value, (c) average value, (d) none of the preceding. (a) It has been proposed that temperature differences in the ocean could be used to run a heat engine to generate electricity. In tropical regions, the water temperature is about 25°C at the surface an ...
pH Scale and Concentration Date: Chemistry!
... It has a higher concentration of H 3O+ than OH– and causes litmus to turn blue. It has a higher concentration of OH – than H3O + and causes litmus to turn blue. It has a higher concentration of H 3O+ than OH– and causes methyl orange to turn yellow. It has a higher concentration of OH – than H 3O+ a ...
... It has a higher concentration of H 3O+ than OH– and causes litmus to turn blue. It has a higher concentration of OH – than H3O + and causes litmus to turn blue. It has a higher concentration of H 3O+ than OH– and causes methyl orange to turn yellow. It has a higher concentration of OH – than H 3O+ a ...
chemistry 1 notes ~ thermochemistry
... A. HEAT (q)—energy flowing from warmer to cooler objects or areas B. THERMOCHEMISTRY 1) the study of heat changes in chemical reactions and physical changes 2) the study of heat flow between a system and its surroundings a. SYSTEM—specific part being analyzed b. SURROUNDINGS—everything outside the s ...
... A. HEAT (q)—energy flowing from warmer to cooler objects or areas B. THERMOCHEMISTRY 1) the study of heat changes in chemical reactions and physical changes 2) the study of heat flow between a system and its surroundings a. SYSTEM—specific part being analyzed b. SURROUNDINGS—everything outside the s ...
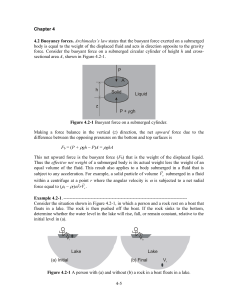
Chapter 4
... Suppose we have a liquid in the bed of a pickup truck that accelerates in the x-direction with constant acceleration ax. The pressure within the fluid is now a function of both x and y since it experiences both the acceleration of gravity in the y-direction and the car acceleration in the x-directio ...
... Suppose we have a liquid in the bed of a pickup truck that accelerates in the x-direction with constant acceleration ax. The pressure within the fluid is now a function of both x and y since it experiences both the acceleration of gravity in the y-direction and the car acceleration in the x-directio ...
Countercurrent exchange

Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some component, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or even solid powders, or any combination of those. For example, in a distillation column, the vapors bubble up through the downward flowing liquid while exchanging both heat and mass.The maximum amount of heat or mass transfer that can be obtained is higher with countercurrent than co-current (parallel) exchange because countercurrent maintains a slowly declining difference or gradient (usually temperature or concentration difference). In cocurrent exchange the initial gradient is higher but falls off quickly, leading to wasted potential. For example, in the diagram at the right, the fluid being heated (exiting top) has a higher exiting temperature than the cooled fluid (exiting bottom) that was used for heating. With cocurrent or parallel exchange the heated and cooled fluids can only approach one another. The result is that countercurrent exchange can achieve a greater amount of heat or mass transfer than parallel under otherwise similar conditions. See: flow arrangement.Countercurrent exchange when set up in a circuit or loop can be used for building up concentrations, heat, or other properties of flowing liquids. Specifically when set up in a loop with a buffering liquid between the incoming and outgoing fluid running in a circuit, and with active transport pumps on the outgoing fluid's tubes, the system is called a Countercurrent multiplier, enabling a multiplied effect of many small pumps to gradually build up a large concentration in the buffer liquid.Other countercurrent exchange circuits where the incoming and outgoing fluids touch each other are used for retaining a high concentration of a dissolved substance or for retaining heat, or for allowing the external buildup of the heat or concentration at one point in the system.Countercurrent exchange circuits or loops are found extensively in nature, specifically in biologic systems. In vertebrates, they are called a Rete mirabile, originally the name of an organ in fish gills for absorbing oxygen from the water. It is mimicked in industrial systems. Countercurrent exchange is a key concept in chemical engineering thermodynamics and manufacturing processes, for example in extracting sucrose from sugar beet roots.Countercurrent multiplication is a similar but different concept where liquid moves in a loop followed by a long length of movement in opposite directions with an intermediate zone. The tube leading to the loop passively building up a gradient of heat (or cooling) or solvent concentration while the returning tube has a constant small pumping action all along it, so that a gradual intensification of the heat or concentration is created towards the loop. Countercurrent multiplication has been found in the kidneys as well as in many other biological organs.