Laws of Thermodynamics
... After the first and second laws of thermodynamics had been recognized and thermodynamics was becoming a more formal subject, it was realized that there was no consistent definition of temperature. A zeroth law of thermodynamics was added to rectify this. Later still, a third law of thermodynamics wa ...
... After the first and second laws of thermodynamics had been recognized and thermodynamics was becoming a more formal subject, it was realized that there was no consistent definition of temperature. A zeroth law of thermodynamics was added to rectify this. Later still, a third law of thermodynamics wa ...
Regular Question Papers
... from the atmosphere. Heat losses through the walls of the house are estimated at 0.65 kW per unit of temperature difference between the inside of house and atmosphere. i) If the atmospheric temperature is -100C, what is the minimum power required to drive the pump? ii) It is proposed to use the same ...
... from the atmosphere. Heat losses through the walls of the house are estimated at 0.65 kW per unit of temperature difference between the inside of house and atmosphere. i) If the atmospheric temperature is -100C, what is the minimum power required to drive the pump? ii) It is proposed to use the same ...
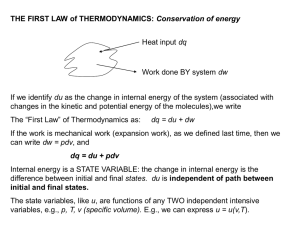
THE FIRST LAW of THERMODYNAMICS: Conservation of energy
... To obey the First Law (du=dw), T must increase. In contrast, T remains constant during the isothermal compression. Since the final temperature is higher in the adiabatic compression, therefore (by Ideal Gas Law), final P must also be higher than for isothermal process: So adiabat is steeper than iso ...
... To obey the First Law (du=dw), T must increase. In contrast, T remains constant during the isothermal compression. Since the final temperature is higher in the adiabatic compression, therefore (by Ideal Gas Law), final P must also be higher than for isothermal process: So adiabat is steeper than iso ...
revision - metc instructors collab site
... when substances at different temperatures are placed in contact they will, in time, reach a common temperature through transfer of heat Defines specific heat capacity as the heat transfer, per unit mass, per unit of temperature change, for any given body or system Uses laboratory equipment to determ ...
... when substances at different temperatures are placed in contact they will, in time, reach a common temperature through transfer of heat Defines specific heat capacity as the heat transfer, per unit mass, per unit of temperature change, for any given body or system Uses laboratory equipment to determ ...
ac nanocalorimeter for measuring heat capacity of biological
... To understand the thermostability of biological macromolecules, proteins and nucleic acids, the study of their heatcapacity function has attracted a great deal of interest because one can obtain a thermodynamic description and prediction of their native state, denatured state and, in some cases, int ...
... To understand the thermostability of biological macromolecules, proteins and nucleic acids, the study of their heatcapacity function has attracted a great deal of interest because one can obtain a thermodynamic description and prediction of their native state, denatured state and, in some cases, int ...
Synthetic Biology: Plasmid Isolation
... 14. Place the QIAprep column in a clean 1.5 ml microcentrifuge tube which has had its lid removed. 15. To elute DNA, add 50 µl water (DEPC treated water) to the center of each QIAprep column, let stand for 1 min and centrifuge for 1 min. 16. The DNA is now ready for further analysis. Store on ice or ...
... 14. Place the QIAprep column in a clean 1.5 ml microcentrifuge tube which has had its lid removed. 15. To elute DNA, add 50 µl water (DEPC treated water) to the center of each QIAprep column, let stand for 1 min and centrifuge for 1 min. 16. The DNA is now ready for further analysis. Store on ice or ...
Consequences of the relation between temperature, heat, and
... For a process where no work is performed (isochoric process), dU = TdS. This gives us another way to define temperature: ...
... For a process where no work is performed (isochoric process), dU = TdS. This gives us another way to define temperature: ...
AP Physics - Thermodynamics
... Heading up the do-not camp was Stuart Nelson Jr., head veterinarian for the famous Iditarod dogsled race currently under way in Alaska. This 1,100-mile event lasts two weeks and features several dozen dog teams and their mushers racing from Anchorage to Nome in some of the most grueling conditions i ...
... Heading up the do-not camp was Stuart Nelson Jr., head veterinarian for the famous Iditarod dogsled race currently under way in Alaska. This 1,100-mile event lasts two weeks and features several dozen dog teams and their mushers racing from Anchorage to Nome in some of the most grueling conditions i ...
CARNOT CYCLE i) substance starts at with temperature T2
... • We need to generalize the definition of entropy since real systems are typically spontaneous and irreversible, moving from a state of non-equilibrium to a state of equilibrium. • Second law can be formulated as 4 postulates: 1. There exists a STATE VARIABLE for any substance called the ENTROPY. ...
... • We need to generalize the definition of entropy since real systems are typically spontaneous and irreversible, moving from a state of non-equilibrium to a state of equilibrium. • Second law can be formulated as 4 postulates: 1. There exists a STATE VARIABLE for any substance called the ENTROPY. ...
Countercurrent exchange

Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some component, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or even solid powders, or any combination of those. For example, in a distillation column, the vapors bubble up through the downward flowing liquid while exchanging both heat and mass.The maximum amount of heat or mass transfer that can be obtained is higher with countercurrent than co-current (parallel) exchange because countercurrent maintains a slowly declining difference or gradient (usually temperature or concentration difference). In cocurrent exchange the initial gradient is higher but falls off quickly, leading to wasted potential. For example, in the diagram at the right, the fluid being heated (exiting top) has a higher exiting temperature than the cooled fluid (exiting bottom) that was used for heating. With cocurrent or parallel exchange the heated and cooled fluids can only approach one another. The result is that countercurrent exchange can achieve a greater amount of heat or mass transfer than parallel under otherwise similar conditions. See: flow arrangement.Countercurrent exchange when set up in a circuit or loop can be used for building up concentrations, heat, or other properties of flowing liquids. Specifically when set up in a loop with a buffering liquid between the incoming and outgoing fluid running in a circuit, and with active transport pumps on the outgoing fluid's tubes, the system is called a Countercurrent multiplier, enabling a multiplied effect of many small pumps to gradually build up a large concentration in the buffer liquid.Other countercurrent exchange circuits where the incoming and outgoing fluids touch each other are used for retaining a high concentration of a dissolved substance or for retaining heat, or for allowing the external buildup of the heat or concentration at one point in the system.Countercurrent exchange circuits or loops are found extensively in nature, specifically in biologic systems. In vertebrates, they are called a Rete mirabile, originally the name of an organ in fish gills for absorbing oxygen from the water. It is mimicked in industrial systems. Countercurrent exchange is a key concept in chemical engineering thermodynamics and manufacturing processes, for example in extracting sucrose from sugar beet roots.Countercurrent multiplication is a similar but different concept where liquid moves in a loop followed by a long length of movement in opposite directions with an intermediate zone. The tube leading to the loop passively building up a gradient of heat (or cooling) or solvent concentration while the returning tube has a constant small pumping action all along it, so that a gradual intensification of the heat or concentration is created towards the loop. Countercurrent multiplication has been found in the kidneys as well as in many other biological organs.