Movement of Fluids and Electrolytes
... of the cell membrane and the specific active transport activity of the cell determine the characteristics of intracellular and extracellular fluid compartments. A profound alteration in anyone of the fluid compartments can disrupt cellular health and may result in a fatal systemic response. Most wat ...
... of the cell membrane and the specific active transport activity of the cell determine the characteristics of intracellular and extracellular fluid compartments. A profound alteration in anyone of the fluid compartments can disrupt cellular health and may result in a fatal systemic response. Most wat ...
Тепломассообмен
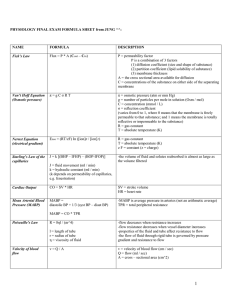
... 4. The property density “p” is defined as the mass per unit volume. Specific volume “v” is the reciprocal of density; that is, v=I/p. Specific gravity “S” is the ratio of the density of a substance to that of pure water at 40C and 76 cm Hg. 5. Temperature “T” is a property which enables us to determ ...
... 4. The property density “p” is defined as the mass per unit volume. Specific volume “v” is the reciprocal of density; that is, v=I/p. Specific gravity “S” is the ratio of the density of a substance to that of pure water at 40C and 76 cm Hg. 5. Temperature “T” is a property which enables us to determ ...
Cumulative Formula Sheet
... -the pressure times volume (at a given t) is constant (diaphragm movement changes lung volume which changes P) ...
... -the pressure times volume (at a given t) is constant (diaphragm movement changes lung volume which changes P) ...
Lecture 5
... We can make a refrigerator by running an engine backwards. Then we do work W taking heat Q1 out of the colder temperature reservoir at T1 , and dumping both W and Q1 into the hot reservoir at T2 . A reasonable definition of the efficiency here is |Q1 | T1 ηc = ...
... We can make a refrigerator by running an engine backwards. Then we do work W taking heat Q1 out of the colder temperature reservoir at T1 , and dumping both W and Q1 into the hot reservoir at T2 . A reasonable definition of the efficiency here is |Q1 | T1 ηc = ...
Experiment 1 - 8. Form of Energy
... heat and mechanical energy is not varied. This is called the conservation of heat and mechanical energy (conservation of total energy), and the mechanical energy conservation exists only in the system where there is no energy variation by the heat. ...
... heat and mechanical energy is not varied. This is called the conservation of heat and mechanical energy (conservation of total energy), and the mechanical energy conservation exists only in the system where there is no energy variation by the heat. ...
Study Questions 1
... What is the function of respiratory pigments? Are the respiratory pigments associated with cells, dissolved in the circulatory fluid or both? Is there any commonality between the respiratory pigments of invertebrates and those of vertebrates? Explain. (NOTE: Except for hemoglobin, you don’t need to ...
... What is the function of respiratory pigments? Are the respiratory pigments associated with cells, dissolved in the circulatory fluid or both? Is there any commonality between the respiratory pigments of invertebrates and those of vertebrates? Explain. (NOTE: Except for hemoglobin, you don’t need to ...
Exercise No. 1 - People(dot)tuke(dot)
... Temperature is one of the basic seven fundamental physical quantities. The scale of thermodynamic temperature is defined by assigning to the triple point of water a temperature of 273,16 K. The SI unit of temperature (Kelvin) is then simply defined as 1/273,16 the temperature of the water triple poi ...
... Temperature is one of the basic seven fundamental physical quantities. The scale of thermodynamic temperature is defined by assigning to the triple point of water a temperature of 273,16 K. The SI unit of temperature (Kelvin) is then simply defined as 1/273,16 the temperature of the water triple poi ...
Post-Midterm Notes
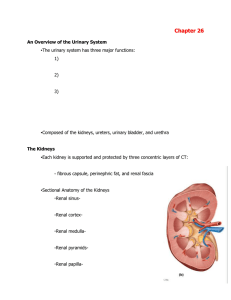
... - renal portal vein is present; carries deoxygenated blood - mammals undergo maximal filtration Mammalian Nephron Function 1. Filtration. Water and low molecular weight substances enter renal capsule. 2. Reabsorption of water and Cl- in proximal tubule by active transport of Na+. 3. Loop of Henle ac ...
... - renal portal vein is present; carries deoxygenated blood - mammals undergo maximal filtration Mammalian Nephron Function 1. Filtration. Water and low molecular weight substances enter renal capsule. 2. Reabsorption of water and Cl- in proximal tubule by active transport of Na+. 3. Loop of Henle ac ...
teaching nerve conduction to undergraduates
... former category. The flame will travel fast if the ignition temperature of the combustible material is low (which would minimize the delay in ignition) and flame temperature is high (which enables a speedier heating of the area adjacent to the flame, since the rate of heat transfer is proportional t ...
... former category. The flame will travel fast if the ignition temperature of the combustible material is low (which would minimize the delay in ignition) and flame temperature is high (which enables a speedier heating of the area adjacent to the flame, since the rate of heat transfer is proportional t ...
Print - Advances in Physiology Education
... former category. The flame will travel fast if the ignition temperature of the combustible material is low (which would minimize the delay in ignition) and flame temperature is high (which enables a speedier heating of the area adjacent to the flame, since the rate of heat transfer is proportional t ...
... former category. The flame will travel fast if the ignition temperature of the combustible material is low (which would minimize the delay in ignition) and flame temperature is high (which enables a speedier heating of the area adjacent to the flame, since the rate of heat transfer is proportional t ...
BCJ0205-15 Thermal phenomena (3-1-4)
... Week 2: Heat, internal energy, heat capacity and specific heat. Latent heat. Week 3: Mechanisms for heat transfer. Week 4: Heat and work in thermodynamic processes. Path between thermodynamic states and the first law of thermodynamics. Week 5: Ideal gases: microscopic interpretation; work performed ...
... Week 2: Heat, internal energy, heat capacity and specific heat. Latent heat. Week 3: Mechanisms for heat transfer. Week 4: Heat and work in thermodynamic processes. Path between thermodynamic states and the first law of thermodynamics. Week 5: Ideal gases: microscopic interpretation; work performed ...
Countercurrent exchange

Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some component, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or even solid powders, or any combination of those. For example, in a distillation column, the vapors bubble up through the downward flowing liquid while exchanging both heat and mass.The maximum amount of heat or mass transfer that can be obtained is higher with countercurrent than co-current (parallel) exchange because countercurrent maintains a slowly declining difference or gradient (usually temperature or concentration difference). In cocurrent exchange the initial gradient is higher but falls off quickly, leading to wasted potential. For example, in the diagram at the right, the fluid being heated (exiting top) has a higher exiting temperature than the cooled fluid (exiting bottom) that was used for heating. With cocurrent or parallel exchange the heated and cooled fluids can only approach one another. The result is that countercurrent exchange can achieve a greater amount of heat or mass transfer than parallel under otherwise similar conditions. See: flow arrangement.Countercurrent exchange when set up in a circuit or loop can be used for building up concentrations, heat, or other properties of flowing liquids. Specifically when set up in a loop with a buffering liquid between the incoming and outgoing fluid running in a circuit, and with active transport pumps on the outgoing fluid's tubes, the system is called a Countercurrent multiplier, enabling a multiplied effect of many small pumps to gradually build up a large concentration in the buffer liquid.Other countercurrent exchange circuits where the incoming and outgoing fluids touch each other are used for retaining a high concentration of a dissolved substance or for retaining heat, or for allowing the external buildup of the heat or concentration at one point in the system.Countercurrent exchange circuits or loops are found extensively in nature, specifically in biologic systems. In vertebrates, they are called a Rete mirabile, originally the name of an organ in fish gills for absorbing oxygen from the water. It is mimicked in industrial systems. Countercurrent exchange is a key concept in chemical engineering thermodynamics and manufacturing processes, for example in extracting sucrose from sugar beet roots.Countercurrent multiplication is a similar but different concept where liquid moves in a loop followed by a long length of movement in opposite directions with an intermediate zone. The tube leading to the loop passively building up a gradient of heat (or cooling) or solvent concentration while the returning tube has a constant small pumping action all along it, so that a gradual intensification of the heat or concentration is created towards the loop. Countercurrent multiplication has been found in the kidneys as well as in many other biological organs.