Lecture 24: Quantum mechanics
... proportional to the intensity of light. Thus Einstein suggested following simple equation: ...
... proportional to the intensity of light. Thus Einstein suggested following simple equation: ...
Problem Set 4
... hydrogen like atom in the presence of an electric field upto second order in Hel given in problem 23 in terms of an infinte sum involving all the eigenstates of the unperturbed hydrogen atom. Estimate the shift using only the n = 2 term ( n is the principal quantum number)in electron volts for the s ...
... hydrogen like atom in the presence of an electric field upto second order in Hel given in problem 23 in terms of an infinte sum involving all the eigenstates of the unperturbed hydrogen atom. Estimate the shift using only the n = 2 term ( n is the principal quantum number)in electron volts for the s ...
Atomic Structure
... a. Bohr postulated that a hydrogen atom could only exist without radiating in one of a set of stationary states. Explain what is meant by this postulate. b. Bohr related his postulate to the classical picture of a hydrogen atom by quantising which quantity? c. Bohr introduced a second postulate to e ...
... a. Bohr postulated that a hydrogen atom could only exist without radiating in one of a set of stationary states. Explain what is meant by this postulate. b. Bohr related his postulate to the classical picture of a hydrogen atom by quantising which quantity? c. Bohr introduced a second postulate to e ...
CHM1045 - Michael Blaber
... E = h * (the relationship between energy and frequency for electromagnetic radiation En = -RH / n2 or En = -B / n2 (the relationship between the energy of an electron in Bohr's model of the hydrogen atom, and the orbit number of the electron) Elevel = RH * (1/ni2 - 1/nf2) or En = B * (1/ni2 - 1/n ...
... E = h * (the relationship between energy and frequency for electromagnetic radiation En = -RH / n2 or En = -B / n2 (the relationship between the energy of an electron in Bohr's model of the hydrogen atom, and the orbit number of the electron) Elevel = RH * (1/ni2 - 1/nf2) or En = B * (1/ni2 - 1/n ...
homework 2, due October 3rd
... In class we discussed the quantization of circular orbits of an electron around a nucleus considering only the Coulomb electrostatic force between the electron and the nucleus. Repeat the argument in the case of a modified interaction between electron and nucleus: assume the potential between them i ...
... In class we discussed the quantization of circular orbits of an electron around a nucleus considering only the Coulomb electrostatic force between the electron and the nucleus. Repeat the argument in the case of a modified interaction between electron and nucleus: assume the potential between them i ...
Chapter 2 Study Guide
... 13. How many energy levels are needed to contain the 26 electrons of an iron atom? __________ 14. The atomic number of calcium is 20. How many valence electrons does an atom of chlorine have? ________ 15. In an electron dot diagram, the dots represent _____________ in the atom. 16. Neutral particle ...
... 13. How many energy levels are needed to contain the 26 electrons of an iron atom? __________ 14. The atomic number of calcium is 20. How many valence electrons does an atom of chlorine have? ________ 15. In an electron dot diagram, the dots represent _____________ in the atom. 16. Neutral particle ...
LECTURE 3 PARTICLE INTERACTIONS & FEYNMAN DIAGRAMS PHY492 Nuclear and Elementary Particle Physics
... ElectromagneGc Weak ...
... ElectromagneGc Weak ...
science 1 small-group tutorial scheme
... List all the subshells and orbitals for which the principal quantum number, n, has the values 2 and 3 in order of increasing energy, according to the quantum mechanical rules. Use clearly-labelled diagrams to illustrate the shapes and orientations of the orbitals for which the angular momentum (subs ...
... List all the subshells and orbitals for which the principal quantum number, n, has the values 2 and 3 in order of increasing energy, according to the quantum mechanical rules. Use clearly-labelled diagrams to illustrate the shapes and orientations of the orbitals for which the angular momentum (subs ...
statpp2006
... • How to combine results from different runs? • Discovered something new? • If not discovery, what we can say from the experiment? Prescriptions to these problems often involve considerations based on statistics. ...
... • How to combine results from different runs? • Discovered something new? • If not discovery, what we can say from the experiment? Prescriptions to these problems often involve considerations based on statistics. ...
Quantum Statistics Applications
... • If want the ratio of number in 2S+2P to 1S to be .1 you need T = 32,000 degrees. (measuring the relative intensity of absorption lines in a star’s atmosphere or a interstellar gas cloud gives T) P460 - Quan. Stats. II ...
... • If want the ratio of number in 2S+2P to 1S to be .1 you need T = 32,000 degrees. (measuring the relative intensity of absorption lines in a star’s atmosphere or a interstellar gas cloud gives T) P460 - Quan. Stats. II ...
Chp.23 Outline - Redlands High School
... 8) Explain the cause of spectral lines produced by an excited gas. What does the color of each line depend upon? Why does each element have its own set of spectral lines? 9) Is an electron more wavelike or particle like? Use this to explain why electrons can only exist at certain distances from the ...
... 8) Explain the cause of spectral lines produced by an excited gas. What does the color of each line depend upon? Why does each element have its own set of spectral lines? 9) Is an electron more wavelike or particle like? Use this to explain why electrons can only exist at certain distances from the ...
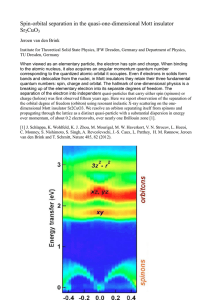
Spin-orbital separation in the quasi-one
... Institute for Theoretical Solid State Physics, IFW Dresden, Germany and Department of Physics, TU Dresden, Germany When viewed as an elementary particle, the electron has spin and charge. When binding to the atomic nucleus, it also acquires an angular momentum quantum number corresponding to the qua ...
... Institute for Theoretical Solid State Physics, IFW Dresden, Germany and Department of Physics, TU Dresden, Germany When viewed as an elementary particle, the electron has spin and charge. When binding to the atomic nucleus, it also acquires an angular momentum quantum number corresponding to the qua ...
Quantum Physics - Particle Physics and Particle Astrophysics
... • but mathematical functions describing particles as waves sometimes give complex numbers • and confined wave packet will disperse over time ...
... • but mathematical functions describing particles as waves sometimes give complex numbers • and confined wave packet will disperse over time ...
5.0. Wave Mechanics
... general validity of eqs(5.1-2). The implied wave-like aspects of “particles” were 1st demonstrated by Thomson and Davisson using the diffraction of electrons by a crystal lattice. The particle-wave duality is best described by the wave mechanical formulism of quantum theory invented by Schrodinger. ...
... general validity of eqs(5.1-2). The implied wave-like aspects of “particles” were 1st demonstrated by Thomson and Davisson using the diffraction of electrons by a crystal lattice. The particle-wave duality is best described by the wave mechanical formulism of quantum theory invented by Schrodinger. ...
The Electron - Student Moodle
... looks like a solar system, with the nucleus in place of the sun and electrons in place of the planets. What actually happens is not that simple. If an electron is in the first energy level (n = 1), the Bohr model may predict its average distance from the nucleus, but the electron is also a particle, ...
... looks like a solar system, with the nucleus in place of the sun and electrons in place of the planets. What actually happens is not that simple. If an electron is in the first energy level (n = 1), the Bohr model may predict its average distance from the nucleus, but the electron is also a particle, ...
Final
... the superconductor. Assuming ∆ ¿ ²F , find the approximate wavefunctions on the normal and superconducting sides, using continuity. [13 mks] (5) Consider the Hamiltonian for an electron system with a 2-body interaction P P p2 † ap ap + p1 ,p2 ,q qvn a†p1 +q a†p2 −q ap2 ap1 , H = p 2m where v is some ...
... the superconductor. Assuming ∆ ¿ ²F , find the approximate wavefunctions on the normal and superconducting sides, using continuity. [13 mks] (5) Consider the Hamiltonian for an electron system with a 2-body interaction P P p2 † ap ap + p1 ,p2 ,q qvn a†p1 +q a†p2 −q ap2 ap1 , H = p 2m where v is some ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.