
Document
... is observed after many electrons have gone through the slits. If we send the electrons through one at a time, we cannot predict the path any single electron will take, but we can predict the overall distribution. ...
... is observed after many electrons have gone through the slits. If we send the electrons through one at a time, we cannot predict the path any single electron will take, but we can predict the overall distribution. ...
Quiz 9
... The atomic number, Z; i.e. the number of protons in the nucleus of the atom determines the ground state energy of an atom. The reason is that the potential experienced by an electron depends on the total charge, hence Z, of the nucleus. 2. In your own words, what are degenerate states. ...
... The atomic number, Z; i.e. the number of protons in the nucleus of the atom determines the ground state energy of an atom. The reason is that the potential experienced by an electron depends on the total charge, hence Z, of the nucleus. 2. In your own words, what are degenerate states. ...
Modeling Single Electron Transistor Sensitivity for Read
... Energy spacing must be greater then thermal smearing ...
... Energy spacing must be greater then thermal smearing ...
Chapter 6 Practice Questions
... 9) According to the Heisenberg Uncertainty Principle, it is impossible to know precisely both the position and the __________ of an electron. A) mass B) color C) momentum D) shape E) charge 10) All of the orbitals in a given electron shell have the same value of the __________ quantum number. A) pri ...
... 9) According to the Heisenberg Uncertainty Principle, it is impossible to know precisely both the position and the __________ of an electron. A) mass B) color C) momentum D) shape E) charge 10) All of the orbitals in a given electron shell have the same value of the __________ quantum number. A) pri ...
Answers to Coursebook questions – Chapter J1
... electrons occupying that state be differentiated in some way. The inner shell has no quantum numbers other than energy, and so the only quantum number that can separate two electrons is the spin. One electron can have spin up and the other spin down. So we can have at most two electrons. In the othe ...
... electrons occupying that state be differentiated in some way. The inner shell has no quantum numbers other than energy, and so the only quantum number that can separate two electrons is the spin. One electron can have spin up and the other spin down. So we can have at most two electrons. In the othe ...
Spectroscopy - Birmingham City Schools
... Maxwell and Boltzman (we’ll hear more about him in future units…) showed that this model explained everything we knew about heat and Temp. o Absorption of EM radiation increases temperature o Reflected EM radiation shows color (if in visible region) But… What controls whether radiation is absorbed ...
... Maxwell and Boltzman (we’ll hear more about him in future units…) showed that this model explained everything we knew about heat and Temp. o Absorption of EM radiation increases temperature o Reflected EM radiation shows color (if in visible region) But… What controls whether radiation is absorbed ...
Microsoft PowerPoint
... predicts the trajectory of an object, whereas the quantum mechanics predicts the probability of an object’s emergence in space. ...
... predicts the trajectory of an object, whereas the quantum mechanics predicts the probability of an object’s emergence in space. ...
Unit_7_Review.noans.annotated
... is 36%. The probability of a student playing football in PE is 38%. 48% of the students played at least one of the two sports during class. • What is the probability of a student playing both sports during the period? ...
... is 36%. The probability of a student playing football in PE is 38%. 48% of the students played at least one of the two sports during class. • What is the probability of a student playing both sports during the period? ...
File - Science With Dumars
... Erwin Schrodinger (mathematical equations using probability, quantum numbers) ...
... Erwin Schrodinger (mathematical equations using probability, quantum numbers) ...
Announcements
... smaller the wavelength of the EM radiation, the more likely the behavior is to be particle-like l The larger the wavelength, the more likely it is to be ...
... smaller the wavelength of the EM radiation, the more likely the behavior is to be particle-like l The larger the wavelength, the more likely it is to be ...
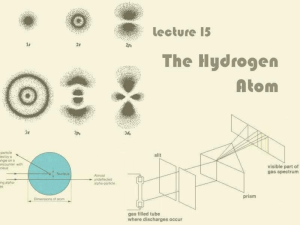
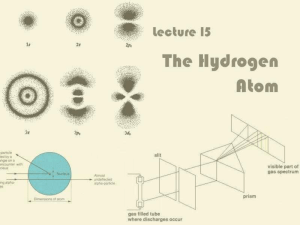
Lecture 15: The Hydrogen Atom
... It only absorbs or emits photons with precisely the right energies dictated by energy conservation ...
... It only absorbs or emits photons with precisely the right energies dictated by energy conservation ...
Quantum Cloud Model
... difference between the two levels (excited and ground state) as electromagnetic radiation with its specific wavelength and frequency ...
... difference between the two levels (excited and ground state) as electromagnetic radiation with its specific wavelength and frequency ...
Lecture
... Configuration: the manner of filling electrons in the orbitals available. There are numerous configurations possible, but there is only one ground state configuration! ...
... Configuration: the manner of filling electrons in the orbitals available. There are numerous configurations possible, but there is only one ground state configuration! ...
qp2
... product of Planck’s constant h and the frequency of the light wave f, that is, E = hf. This crucial equation relates the wave property of light (its wave frequency) to the particle property of light (energy packet), thus showing that light behaves as both particle and wave. The next riddle related ...
... product of Planck’s constant h and the frequency of the light wave f, that is, E = hf. This crucial equation relates the wave property of light (its wave frequency) to the particle property of light (energy packet), thus showing that light behaves as both particle and wave. The next riddle related ...
lecture notes, page 1
... 1913 Niels Bohr (Danish scientist) predicted quantized levels for H atom prior to QM development, with the electron in welldefined circular orbits. This was still a purely particle picture of the e–. ...
... 1913 Niels Bohr (Danish scientist) predicted quantized levels for H atom prior to QM development, with the electron in welldefined circular orbits. This was still a purely particle picture of the e–. ...
Physical Chemistry II – Exam 1 SOLUTIONS
... Explain the concept of quantization. Then, using one example system, describe at least one property of the system that exhibits quantization. Make sure to include in your discussion an equation that illustrates quantization of the property of interest. Quantization is the idea that a physical proper ...
... Explain the concept of quantization. Then, using one example system, describe at least one property of the system that exhibits quantization. Make sure to include in your discussion an equation that illustrates quantization of the property of interest. Quantization is the idea that a physical proper ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.



















![NAME: Quiz #5: Phys142 1. [4pts] Find the resulting current through](http://s1.studyres.com/store/data/006404813_1-90fcf53f79a7b619eafe061618bfacc1-300x300.png)
