Chem 121 QU 78 Due in lecture
... →__________________________________________________________________________ Write the electron configuration diagram on the above blank↑ → n = _______ l = _______ Write the 2 quantum numbers for an electron indicated by the above arrow. ...
... →__________________________________________________________________________ Write the electron configuration diagram on the above blank↑ → n = _______ l = _______ Write the 2 quantum numbers for an electron indicated by the above arrow. ...
Slide 1
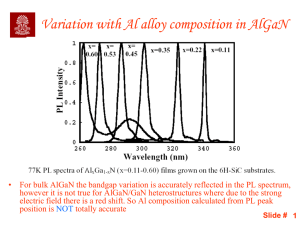
... • For bulk AlGaN the bandgap variation is accurately reflected in the PL spectrum, however it is not true for AlGaN/GaN heterostructures where due to the strong electric field there is a red shift. So Al composition calculated from PL peak position is NOT totally accurate ...
... • For bulk AlGaN the bandgap variation is accurately reflected in the PL spectrum, however it is not true for AlGaN/GaN heterostructures where due to the strong electric field there is a red shift. So Al composition calculated from PL peak position is NOT totally accurate ...
Document
... • An electron is characterized by quantum numbers. These can be measured without uncertainty. • The quantum number n labels the energy level En . • The lowest energy level with n = 1 is sharp (E= 0), because an atom is stable. One can take an infinite time (t = ) to determine its energy and there ...
... • An electron is characterized by quantum numbers. These can be measured without uncertainty. • The quantum number n labels the energy level En . • The lowest energy level with n = 1 is sharp (E= 0), because an atom is stable. One can take an infinite time (t = ) to determine its energy and there ...
Unit 4 review sheet
... 35. Draw Lewis electron dot diagrams for the elements in problem 12. 36. Heisenberg stated that, at the same time, it was impossible to know what two things about the electron? 37. How many quantum numbers are there? 38. What letter denotes the quantum number for the principle energy level? 39. What ...
... 35. Draw Lewis electron dot diagrams for the elements in problem 12. 36. Heisenberg stated that, at the same time, it was impossible to know what two things about the electron? 37. How many quantum numbers are there? 38. What letter denotes the quantum number for the principle energy level? 39. What ...
INFORMATION ON MASTER`S THESIS 1. Full name: VU THI MINH
... 11. Summary of the finding of the thesis: Thesis: “Anomalous magnetic moment of electrons and methods Pauli – Villars in quantum field theory” study the anomalous magnetic moment of the electron in quantum field theory. The main complementary magnetic moment based on perturbation theory via covarian ...
... 11. Summary of the finding of the thesis: Thesis: “Anomalous magnetic moment of electrons and methods Pauli – Villars in quantum field theory” study the anomalous magnetic moment of the electron in quantum field theory. The main complementary magnetic moment based on perturbation theory via covarian ...
The Transactional Interpretation
... small particles such as atoms, electrons, photons, and other subatomic particles. • QM works very well but what it actually tells us about reality is very unclear • An interpretation is intended to make clear what the theory tells us about reality ...
... small particles such as atoms, electrons, photons, and other subatomic particles. • QM works very well but what it actually tells us about reality is very unclear • An interpretation is intended to make clear what the theory tells us about reality ...
PPT - Lawless Teaching : Home
... all other charges are integer multiples of this (we will later see quarks as the exception to this). Named by an Irish guy, George J. Stoney (cause that’s important). ...
... all other charges are integer multiples of this (we will later see quarks as the exception to this). Named by an Irish guy, George J. Stoney (cause that’s important). ...
Electrons in Atoms
... that since we don’t know where exactly an electron is at any given moment, it is actually in all possible states simultaneously, as long as we don't look to check. It is the measurement itself that causes the object to be limited to a single possibility. ...
... that since we don’t know where exactly an electron is at any given moment, it is actually in all possible states simultaneously, as long as we don't look to check. It is the measurement itself that causes the object to be limited to a single possibility. ...
Electron Configuration and New Atomic Model
... • A form of energy that exhibits wavelike behavior as it travels through space is called electromagnetic radiation. • All forms of this radiation make up the electromagnetic spectrum. • All forms move at the speed of light (c= 3.0 x108 m/s), through a vacuum and slightly slower through matter. • ...
... • A form of energy that exhibits wavelike behavior as it travels through space is called electromagnetic radiation. • All forms of this radiation make up the electromagnetic spectrum. • All forms move at the speed of light (c= 3.0 x108 m/s), through a vacuum and slightly slower through matter. • ...
Exercise 1, from the final exam in AST4220, 2005 Exercise 2
... where H0 is the Hubble constant. Calculate the value of t0 for H0 = 70 km s−1 Mpc−1 . Use this model and this value for H0 in the remainder of this problem. c) Calculate tdec , the age of the Univere at zdec . d) Calculate dPH (zdec ), the proper distance to the particle horizon at tdec . e) Calcula ...
... where H0 is the Hubble constant. Calculate the value of t0 for H0 = 70 km s−1 Mpc−1 . Use this model and this value for H0 in the remainder of this problem. c) Calculate tdec , the age of the Univere at zdec . d) Calculate dPH (zdec ), the proper distance to the particle horizon at tdec . e) Calcula ...
Problems
... 3. Show that the spectral density, uω (equation 1.2.4) peaks at Eph = 2.82 kT. Note that a numeric iteration is required. 4. Calculate the peak wavelength of blackbody radiation emitted from a human body at a temperature of 37°C. 5. Derive equations (1.2.9) and (1.2.10). 6. What is the width of an i ...
... 3. Show that the spectral density, uω (equation 1.2.4) peaks at Eph = 2.82 kT. Note that a numeric iteration is required. 4. Calculate the peak wavelength of blackbody radiation emitted from a human body at a temperature of 37°C. 5. Derive equations (1.2.9) and (1.2.10). 6. What is the width of an i ...
Syllabus
... The main objective of this course is to examine the theoretical basis for our present understanding of the structure of matter at the atomic and molecular level. To that end we will review those aspects of quantum mechanics that play the most important role in this understanding. This includes the s ...
... The main objective of this course is to examine the theoretical basis for our present understanding of the structure of matter at the atomic and molecular level. To that end we will review those aspects of quantum mechanics that play the most important role in this understanding. This includes the s ...
Electrons in Atoms 5.1 Worksheet
... Niels Bohr proposed that electrons move in specific orbits around the nucleus. In these orbits, each electron has a fixed energy called an energy level. A quantum of energy is the amount of energy needed to move an electron from one energy level to another. The Quantum Mechanical Model The quantum m ...
... Niels Bohr proposed that electrons move in specific orbits around the nucleus. In these orbits, each electron has a fixed energy called an energy level. A quantum of energy is the amount of energy needed to move an electron from one energy level to another. The Quantum Mechanical Model The quantum m ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.