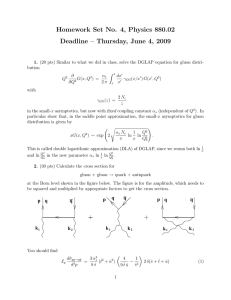
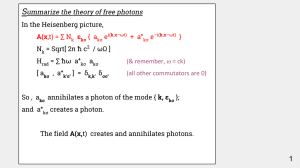
Quantum Theory of Hydrogen
... who does anything relating to modern physics and puts class material on the web seems to do the hydrogen atom. A search using Google for “hydrogen atom schrodinger equation” (but don’t put the words in quotes when you do the search) comes up with about 4300 sites. Many of them are much nicer looking ...
... who does anything relating to modern physics and puts class material on the web seems to do the hydrogen atom. A search using Google for “hydrogen atom schrodinger equation” (but don’t put the words in quotes when you do the search) comes up with about 4300 sites. Many of them are much nicer looking ...
Quantum Theory
... Hot objects emit light and other forms of electromagnetic radiation, but not continuously, as expected Instead, it is emitted in small, specific amounts called quanta. E=hv H=6.626 x 10 -34 J*s This number is called Planck’s constant ...
... Hot objects emit light and other forms of electromagnetic radiation, but not continuously, as expected Instead, it is emitted in small, specific amounts called quanta. E=hv H=6.626 x 10 -34 J*s This number is called Planck’s constant ...
example8
... (b) The photoelectric effect is the emission of electrons from a metal surface when electromagnetic radiation is shone onto the metal. For this to happen the frequency of the electromagnetic radiation must be above a certain minimum frequency known as the threshold frequency. If the frequency is bel ...
... (b) The photoelectric effect is the emission of electrons from a metal surface when electromagnetic radiation is shone onto the metal. For this to happen the frequency of the electromagnetic radiation must be above a certain minimum frequency known as the threshold frequency. If the frequency is bel ...
Chapter 2
... 9. Given the following sets of quantum numbers, set up the orbital notation (labeled with the sublevel designation) for the indicated sublevel of electrons and circle the indicated orbital. Assume that electrons fill from more negative to more positive ml values. ...
... 9. Given the following sets of quantum numbers, set up the orbital notation (labeled with the sublevel designation) for the indicated sublevel of electrons and circle the indicated orbital. Assume that electrons fill from more negative to more positive ml values. ...
Wave as particle 2
... When photon with energy above the rest mass of two electrons ( 2me c 2 ) interact with the electric field of a nucleus, this photon may be turned into a pair of electron and positron. This process is called pair production through which energy gets turned into mass. Positron is the anti-particle of ...
... When photon with energy above the rest mass of two electrons ( 2me c 2 ) interact with the electric field of a nucleus, this photon may be turned into a pair of electron and positron. This process is called pair production through which energy gets turned into mass. Positron is the anti-particle of ...
LAMB SHIFT & VACUUM POLARIZATION CORRECTIONS TO THE
... This result is formally divergent, but there are physical factors that cut off the integral. At large frequencies there occurs the relativistic growth of the electrons mass; for small frequencies the perturbation does not work if h̄ω ¿ distance to the first excited state. The divergence is only log ...
... This result is formally divergent, but there are physical factors that cut off the integral. At large frequencies there occurs the relativistic growth of the electrons mass; for small frequencies the perturbation does not work if h̄ω ¿ distance to the first excited state. The divergence is only log ...
Quantum Mechanics and the Bohr Model - slater science
... waves superimposed on each other. Both waves should have the same amplitude but different frequencies. • Draw another horizontal line and two waves with the same wavelength but different amplitudes. ...
... waves superimposed on each other. Both waves should have the same amplitude but different frequencies. • Draw another horizontal line and two waves with the same wavelength but different amplitudes. ...
Slide 1
... The Puzzle of the Atom Protons and electrons are attracted to each other because of opposite charges Electrically charged particles moving in a curved path give off energy ...
... The Puzzle of the Atom Protons and electrons are attracted to each other because of opposite charges Electrically charged particles moving in a curved path give off energy ...
The length of photon
... and measurements. This trend requires precise determination of fundamental physical quantities. Many manufacturing methods, such as laser processing or photolithography, are based on precisely controlled beam of light and hence the quantum of this energy – photon. There is no doubt that the photon s ...
... and measurements. This trend requires precise determination of fundamental physical quantities. Many manufacturing methods, such as laser processing or photolithography, are based on precisely controlled beam of light and hence the quantum of this energy – photon. There is no doubt that the photon s ...
7.4 The Wave Nature of Matter * 7.5 Quantum Mechanics and the Atom
... Electrons (Particles or Waves?) • Electrons exhibit both particle and wave nature (wave-particle duality). • Heisenberg’s uncertainty principle: we are unable to identify a particles position and velocity at the same time. • Since we can not determine the exact location and velocity of an electron ...
... Electrons (Particles or Waves?) • Electrons exhibit both particle and wave nature (wave-particle duality). • Heisenberg’s uncertainty principle: we are unable to identify a particles position and velocity at the same time. • Since we can not determine the exact location and velocity of an electron ...
S
... WKAR-FM is a public radio station in East Lansing, Michigan; broadcasting on the FM dial at 90.5 MHz. It is owned by Michigan State University, and is sister station to the AM radio and television stations with the same call letters. The station signed on for the first time on October 4, 1948 as the ...
... WKAR-FM is a public radio station in East Lansing, Michigan; broadcasting on the FM dial at 90.5 MHz. It is owned by Michigan State University, and is sister station to the AM radio and television stations with the same call letters. The station signed on for the first time on October 4, 1948 as the ...
CHEM 121
... Like the de Broglie relation, the Heisenberg Uncertainty Principle is important only for very small masses such as e-s, n0s, etc. §8-6 Quantum Mechanics & Schrödinger equation: Bohr model only applies to atoms with one eMotivated by classical wave equation for violin string ...
... Like the de Broglie relation, the Heisenberg Uncertainty Principle is important only for very small masses such as e-s, n0s, etc. §8-6 Quantum Mechanics & Schrödinger equation: Bohr model only applies to atoms with one eMotivated by classical wave equation for violin string ...
CHM 441: QUANTUM CHEMISTRY
... which marks the beginning of quantum mechanics applied to atoms, but was unable to describe atoms with more than one electron. ...
... which marks the beginning of quantum mechanics applied to atoms, but was unable to describe atoms with more than one electron. ...
Lecture 1
... closely specified nature which must be assigned to each isolated portion of energy and which depends on its eigenmass in accordance with the PlanckEinstein relation. Then the relativity theory requires the assignment to the uniform movement of each material point of the propagation of a certain wave ...
... closely specified nature which must be assigned to each isolated portion of energy and which depends on its eigenmass in accordance with the PlanckEinstein relation. Then the relativity theory requires the assignment to the uniform movement of each material point of the propagation of a certain wave ...
WP1
... So we cant have both the knowledge of which slit the electron went thru AND have the interference pattern, so how can we ever know which slit it went thru when there IS a wave-like pattern? Maybe we can NEVER know? So, QM postulates that there two wavelike phenomena (one from each slit) that interf ...
... So we cant have both the knowledge of which slit the electron went thru AND have the interference pattern, so how can we ever know which slit it went thru when there IS a wave-like pattern? Maybe we can NEVER know? So, QM postulates that there two wavelike phenomena (one from each slit) that interf ...
Orbitals and Quantum Numbers
... l can take on integral values from 0 to n-1 for each value of n ...
... l can take on integral values from 0 to n-1 for each value of n ...
Constructive Interference
... The wavefunction Mathematically, we describe a quantum superposition by a function Y(x) ...
... The wavefunction Mathematically, we describe a quantum superposition by a function Y(x) ...
Quantum phenomena
... • whatever happens in between emission and absorption, light is always emitted and absorbed in discrete amounts of energy. Einstein: ‘God does not play dice.’ Feynman: ‘things on a small scale behave nothing like things on a ...
... • whatever happens in between emission and absorption, light is always emitted and absorbed in discrete amounts of energy. Einstein: ‘God does not play dice.’ Feynman: ‘things on a small scale behave nothing like things on a ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.