* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Quantum Numbers
Renormalization group wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Dirac equation wikipedia , lookup
Renormalization wikipedia , lookup
Particle in a box wikipedia , lookup
History of quantum field theory wikipedia , lookup
Double-slit experiment wikipedia , lookup
Ferromagnetism wikipedia , lookup
EPR paradox wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Chemical bond wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Matter wave wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Wave–particle duality wikipedia , lookup
Tight binding wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Molecular orbital wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Atomic theory wikipedia , lookup
Electron-beam lithography wikipedia , lookup
Hydrogen atom wikipedia , lookup
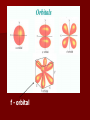
The Quantum Model of the Atom Honors Chemistry Louie de Broglie Proposed that all particles of matter that move exhibit wave like behavior (even a baseball!) He came up with the following equation that relates the wavelength of a particle to its mass and velocity. (Derived from E=mc2 and E = hc/) = h_ mv Using the mass of an electron moving at the speed of light, he calculated the same energy level values as Bohr did for the Hydrogen atom. Louis de Broglie De Broglie realized that the electrons exhibit wavelike properties in their quantized orbits. (draw pictures ) He said that if an electron has wavelike motion and is restricted to circular orbits of a fixed radius, the electron is allowed only certain possible wavelengths, frequencies and energies. Experiments did show that electrons in atoms do exhibit wave behavior with specific frequencies. The Heisenberg Uncertainty Principle Heisenberg concluded that it is impossible to make any measurement on an object without disturbing it – at least a little. Electrons are detected by photons and because a photon and an electron have the same energy, any attempt to locate an electron with a photon will knock the electron off course. Therefore: It is impossible to know both the position and the velocity of an electron at the same time. So we can only talk about the probability of finding and electron in certain area (remember the fuzzy cloud!) Erwin Schrodinger Used the idea that electrons behave like waves to write and solve a mathematical equation to describe the location and energy of an electron in the hydrogen atom. The modern description of the electron cloud in atoms comes from the solutions to the Schrodinger equation. This equation showed that the energy of electrons are restricted to certain values. Erwin Schrodinger However, the equation does not define the exact path the electron takes around the nucleus. It only estimates the probability of finding an electron in a certain position, unlike Bohr’s circular orbits. Electrons exist in regions called Orbitals Orbital – 3 dimensional cloud shape around the nucleus that indicates the probable location of an electron Atomic Orbitals The solutions to the Schrodinger equation are called Quantum Numbers, and they are used to describe the properties, such as the energy level and shape, of the atomic orbitals. “s” - orbital “p” orbitals “d” orbitals f - orbital Quantum Number Information Definition: Set of 4 numbers that tells us the probable location of the electron. It is like a zip code for the electron The numbers tell us the energy level, shape and orientation of the orbital And, it tells us the spin direction of the electron in that orbital Quantum Number Information Name Definition Symbol Assigned Value Principle Quantum Number Indicates the main energy level occupied by the electron Secondary Quantum Number Indicates the shape of the orbital occupied by the electron Magnetic Quantum Number Spin Quantum Number Indicates the orientation of the orbital around the nucleus m If l = 1 Then m = -1, 0 , +1 Indicates the spin direction of the electron. s +½ or -½ n l n = 1, 2, 3, . . . 7. . l l l l = 0 to (n-1) = 0 “s” orbital = 1 “p” orbital = 2 “d” orbital m = -l to +l Only 2 electrons are allowed in each orbital Each with an opposite spin. Given the following quantum numbers, describe the “probable” location of the electron. n, l, m, s, 2, 1, 0, + ½ The electron is in energy level 2 It is in a “p” orbital The orientation of the orbital is the y-axis Quantum # and Atomic Structure Main Energy Level (n) Sublevels (n) *Orbital shapes # of orbitals per sublevel 1 s 2 s p 1 3 3 s p d 1 3 5 4 s p d f 1 3 5 7 1 # orbitals per main energy level (n2) # of electrons per sublevel Total # of electrons per main energy level (2n2) 1 2 2 4 s= 2 p= 6 8 9 s= 2 p=6 d = 10 18 16 s=2 p=6 d = 10 f = 14 32