7th Grade Atomic Structure and Periodic Table of Elements
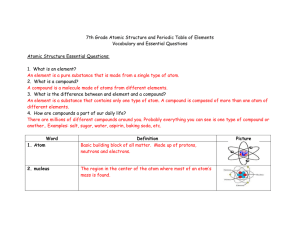
... An element is a pure substance that is made from a single type of atom. 2. What is a compound? A compound is a molecule made of atoms from different elements. 3. What is the difference between and element and a compound? An element is a substance that contains only one type of atom. A compound is co ...
... An element is a pure substance that is made from a single type of atom. 2. What is a compound? A compound is a molecule made of atoms from different elements. 3. What is the difference between and element and a compound? An element is a substance that contains only one type of atom. A compound is co ...
Atoms Introduction Notes and Vocabulary
... Introduction to Atoms- Vocabulary and Notes ATOM - smallest piece of an element that is still that element ELEMENT - matter that is made of only ONE type of atoms. Ex.: Iron, Silver, Carbon, Gold, Oxygen, and others located on the Periodic Table of Elements. PROTON – positively charged particle foun ...
... Introduction to Atoms- Vocabulary and Notes ATOM - smallest piece of an element that is still that element ELEMENT - matter that is made of only ONE type of atoms. Ex.: Iron, Silver, Carbon, Gold, Oxygen, and others located on the Periodic Table of Elements. PROTON – positively charged particle foun ...
ChemFinalgeocities
... Which of the following has the greatest density? a. a rock c. oil b. oxygen d. ice A 26.0-g sample of a liquid was found to have a volume of 13.0 mL. What is the density of the liquid? a. 0.500 g/mL c. 39.0 g/mL b. 2.00 g/mL d. 338 g/mL Coal burns in a furnace, producing light and heat. This reactio ...
... Which of the following has the greatest density? a. a rock c. oil b. oxygen d. ice A 26.0-g sample of a liquid was found to have a volume of 13.0 mL. What is the density of the liquid? a. 0.500 g/mL c. 39.0 g/mL b. 2.00 g/mL d. 338 g/mL Coal burns in a furnace, producing light and heat. This reactio ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... The atomic model has changed over time. For over two centuries, scientists have created different models of the atom. ...
... The atomic model has changed over time. For over two centuries, scientists have created different models of the atom. ...
Slide 1
... 19. Which set of elements contains a metalloid? 1. K, Mn, As, Ar 2. Li, Mg, Ca, Kr 3. Ba, Ag, Sn, Xe 4. Fr, F, O, Rn ...
... 19. Which set of elements contains a metalloid? 1. K, Mn, As, Ar 2. Li, Mg, Ca, Kr 3. Ba, Ag, Sn, Xe 4. Fr, F, O, Rn ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... The atomic model has changed over time. For over two centuries, scientists have created different models of the atom. ...
... The atomic model has changed over time. For over two centuries, scientists have created different models of the atom. ...
Review Questions
... 5. Find the percent composition of Oxygen in Na2S2O3 __________________________ ...
... 5. Find the percent composition of Oxygen in Na2S2O3 __________________________ ...
Periodic Table ppt
... atom is also the Atomic number, so therefore the Atomic number also represents the amount of Protons in the nucleus of that Atom. ...
... atom is also the Atomic number, so therefore the Atomic number also represents the amount of Protons in the nucleus of that Atom. ...
Atomic Structure and the Periodic Table
... Scientists once thought these metals were available only in tiny amounts on the Earth ...
... Scientists once thought these metals were available only in tiny amounts on the Earth ...
MatterPP4
... elements that are chemically combined. Most compounds have totally different properties from the elements of which they are composed. Chemical bonds are the forces that hold the elements together in a compound creating a state of stability. ...
... elements that are chemically combined. Most compounds have totally different properties from the elements of which they are composed. Chemical bonds are the forces that hold the elements together in a compound creating a state of stability. ...
Elements and Atoms
... 1. Protons (P) = positively charged particles 2. Neutrons (N) = no charge (neutral neutral) neutral ...
... 1. Protons (P) = positively charged particles 2. Neutrons (N) = no charge (neutral neutral) neutral ...
PowerPoint - Models of the Atom - A Historical Perspective
... Q- Draw B-R diagrams for the two Li isotopes. A- The atomic # of an element doesn’t change Although the number of neutrons can vary, atoms have definite numbers of protons. ...
... Q- Draw B-R diagrams for the two Li isotopes. A- The atomic # of an element doesn’t change Although the number of neutrons can vary, atoms have definite numbers of protons. ...
Atomic Structure and Periodic Table Review Guide
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
Oxidation-Reduction (Redox) Reactions
... In a compound or as an ion, alkali metals will always be +1. In a compound or an ion, alkaline metals will always be +2. (The other atoms in the compound have to adjust their oxidation states to give the correct overall charge. e.g. NaH) O is –2 (unless two Os bonded to each other) (Everything else ...
... In a compound or as an ion, alkali metals will always be +1. In a compound or an ion, alkaline metals will always be +2. (The other atoms in the compound have to adjust their oxidation states to give the correct overall charge. e.g. NaH) O is –2 (unless two Os bonded to each other) (Everything else ...
Primary electrons make random elastic and inelastic collision either
... effect…. i.e. as to pass though the stronger electric filed, close to nuclei, it will suffer a “quantum jump” to a low energy state, which will make emission of X-ray photon, and it would be all possible energy up to E0… Secondary electron, (<50 eV, normally around 2-6 eV, larger than sample’s work ...
... effect…. i.e. as to pass though the stronger electric filed, close to nuclei, it will suffer a “quantum jump” to a low energy state, which will make emission of X-ray photon, and it would be all possible energy up to E0… Secondary electron, (<50 eV, normally around 2-6 eV, larger than sample’s work ...
ch3 B - Manasquan Public Schools
... move around atoms in set paths around the nucleus. He said each path is an energy level ...
... move around atoms in set paths around the nucleus. He said each path is an energy level ...
Units 3 and 4 Revision
... Q4. Explain why the metal elements in group 1 are (a) called the alkali metals. (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
... Q4. Explain why the metal elements in group 1 are (a) called the alkali metals. (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
Parts of an Atom
... The period an element is in is equal to the number of energy levels it has. (see the column of numbers in the upper right hand corner of each box) Properties change slowly from one end of each row to the other. Groups: Vertical columns of elements All elements in a group have similar propertie ...
... The period an element is in is equal to the number of energy levels it has. (see the column of numbers in the upper right hand corner of each box) Properties change slowly from one end of each row to the other. Groups: Vertical columns of elements All elements in a group have similar propertie ...
O 2 (g)
... • Consider the three metals Li, Na, and K – All 3 metals are soft – All 3 metals are less dense than water – All 3 metals have similar appearance and low melting points – The most interesting feature is that all 3 metals react with the same elements in a nearly identical manner • As you see in the p ...
... • Consider the three metals Li, Na, and K – All 3 metals are soft – All 3 metals are less dense than water – All 3 metals have similar appearance and low melting points – The most interesting feature is that all 3 metals react with the same elements in a nearly identical manner • As you see in the p ...
Atomic Structure and the Periodic Table
... number of positive charges equal the number of negative charges All elements on periodic table are stable atoms. ...
... number of positive charges equal the number of negative charges All elements on periodic table are stable atoms. ...
Posttest answers - Aurora City Schools
... An atom with a different number of electrons, so it has a charge (happens during chemical reactions). It’s written with the charge in the upper right hand corner 59. - 60. What is an isotope and how is the symbol written differently? ...
... An atom with a different number of electrons, so it has a charge (happens during chemical reactions). It’s written with the charge in the upper right hand corner 59. - 60. What is an isotope and how is the symbol written differently? ...