First 9 weeks Study Guide 8th Grade
... Ductile – ability to be hammered thin or turn into wire: Copper has high ductility. ...
... Ductile – ability to be hammered thin or turn into wire: Copper has high ductility. ...
Periodic Trends
... These are the electrons on the outermost ring. They are available for bonding. They can be found by counting the columns on the Periodic Table. EX: P in group 5A = 5 valence electrons ...
... These are the electrons on the outermost ring. They are available for bonding. They can be found by counting the columns on the Periodic Table. EX: P in group 5A = 5 valence electrons ...
Earth`s Chemistry
... there’s no charge. It’s said to be neutral Example --- Oxygen has an atomic number of 8 so it has 8 protons & 8 electrons mass number = protons + neutrons Protons & neutrons have an atomic mass of 1 ...
... there’s no charge. It’s said to be neutral Example --- Oxygen has an atomic number of 8 so it has 8 protons & 8 electrons mass number = protons + neutrons Protons & neutrons have an atomic mass of 1 ...
CHAPTER 3 sec 1 - Leon County Schools
... one element are different from another element. 3. Atoms of different elements can physically mix together (chemically combine) in whole-number ratios to form compounds. ...
... one element are different from another element. 3. Atoms of different elements can physically mix together (chemically combine) in whole-number ratios to form compounds. ...
rocks and minerals quiz
... An atom is mostly empty space between the electrons and the nucleus. This presents a conceptual problem: How do atoms form solids with all this empty space? ATOMIC STRUCTURE ANALOGY Imagine a jungle gym on a children’s playground. If you are a bug up close, you would see a large jungle gym with plen ...
... An atom is mostly empty space between the electrons and the nucleus. This presents a conceptual problem: How do atoms form solids with all this empty space? ATOMIC STRUCTURE ANALOGY Imagine a jungle gym on a children’s playground. If you are a bug up close, you would see a large jungle gym with plen ...
Chapter 4 4.1 Defining the Atom • Early Models of the Atom atom
... October 14, 2016 4.2 Structure of the Nuclear Atom • Subatomic particles ...
... October 14, 2016 4.2 Structure of the Nuclear Atom • Subatomic particles ...
Bill Nye Atoms Workseet
... The main difference between aluminum and copper is the ___________________ of ___________________ in the nucleus. Scientists have grouped all the different elements into groups which have come to be called ________________ The atomic number is the number of _________________________ in the _________ ...
... The main difference between aluminum and copper is the ___________________ of ___________________ in the nucleus. Scientists have grouped all the different elements into groups which have come to be called ________________ The atomic number is the number of _________________________ in the _________ ...
Vocabulary and Section Summary
... Name ______________________________ Class___________________Date__________________ ...
... Name ______________________________ Class___________________Date__________________ ...
Topic 3&4 Atoms and the per.table
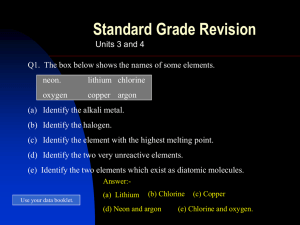
... Q4. Explain why the metal elements in group 1 are (a) called the alkali metals. (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
... Q4. Explain why the metal elements in group 1 are (a) called the alkali metals. (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
Concepts of Physical Science
... 11. A vertical column on the periodic table; numbered from 1 to 18 12. Exhibit both metallic and nonmetallic properties; eight elements (B, Si, Ge, As, Sb, Te, Po, At) 13. A horizontal row on the periodic table numbered 1 through 7 14. any electron in the outermost occupied shell of an atom. 15. th ...
... 11. A vertical column on the periodic table; numbered from 1 to 18 12. Exhibit both metallic and nonmetallic properties; eight elements (B, Si, Ge, As, Sb, Te, Po, At) 13. A horizontal row on the periodic table numbered 1 through 7 14. any electron in the outermost occupied shell of an atom. 15. th ...
What does an elements atomic mass tell us about the element?
... Potassium - K Atomic # = 19 Mass # = 39 K nucleus contains 19 protons 39 – 19 = 20 neutrons How many electrons? Same as # Protons (19) ...
... Potassium - K Atomic # = 19 Mass # = 39 K nucleus contains 19 protons 39 – 19 = 20 neutrons How many electrons? Same as # Protons (19) ...
C1.1 The fundamental ideas in chemistry
... oxygen. Candidates are not required to know of trends within each group in the periodic table, but should be aware of similarities between the elements within a group. The elements in Group 0 of the periodic table are called the noble gases. They are unreactive because their atoms have stable arrang ...
... oxygen. Candidates are not required to know of trends within each group in the periodic table, but should be aware of similarities between the elements within a group. The elements in Group 0 of the periodic table are called the noble gases. They are unreactive because their atoms have stable arrang ...
Chapter 18 Atoms and Elements
... * Atoms of the same element ________ have different number of ___________, which means the mass can vary. 2. Atomic Mass- the average of ________________ of an element. ...
... * Atoms of the same element ________ have different number of ___________, which means the mass can vary. 2. Atomic Mass- the average of ________________ of an element. ...
Ionization energy
... Reflection of how strongly an atom holds onto its outermost electron. Atoms with high ionization energies hold onto their electrons very tightly. Atoms with low ionization energies are more likely to lose one or more of their outermost electron. ...
... Reflection of how strongly an atom holds onto its outermost electron. Atoms with high ionization energies hold onto their electrons very tightly. Atoms with low ionization energies are more likely to lose one or more of their outermost electron. ...
Shiny, Happy Pretest - Alex LeMay – Science
... ___29. What is the atomic number? ___30. What is the atomic mass? ___31. How many protons would an atom of this element have? ___32. How many neutrons would an atom of this element likely have? ___33. How many electrons would an atom of this element have? ___34. If an atom of this element had 30 neu ...
... ___29. What is the atomic number? ___30. What is the atomic mass? ___31. How many protons would an atom of this element have? ___32. How many neutrons would an atom of this element likely have? ___33. How many electrons would an atom of this element have? ___34. If an atom of this element had 30 neu ...
Atomic Size - ThinkChemistry
... The purpose of this activity is to examine how atomic size changes on going down a column in the periodic table and also on going across a row. Going Down a Group (column): The size of an atom increases going down a group. This is because on going down the group from one element to the next, an elec ...
... The purpose of this activity is to examine how atomic size changes on going down a column in the periodic table and also on going across a row. Going Down a Group (column): The size of an atom increases going down a group. This is because on going down the group from one element to the next, an elec ...
The Chemical Basis of Life
... Ionic Bonds (continued) When the electron moves from one atom to another, the atoms now become ...
... Ionic Bonds (continued) When the electron moves from one atom to another, the atoms now become ...
The atom - WordPress.com
... In 1910 JJ Thomson discovered that even atoms from the same element varied in mass. He called atoms from the same element with varying masses isotopes. The average of all the known isotopes of an element give the element its average atomic mass. Elements on the periodic table have decimals in their ...
... In 1910 JJ Thomson discovered that even atoms from the same element varied in mass. He called atoms from the same element with varying masses isotopes. The average of all the known isotopes of an element give the element its average atomic mass. Elements on the periodic table have decimals in their ...
AlBr3 E IO Ionic FU C O Cov Molec C IO Cov Molec Sn E N/A N/A
... old bonds between atoms are broken down and new bonds are formed. Atoms, however, can be created or destroyed in nuclear reactions: radioactive decays, nuclear fission and fusion. ...
... old bonds between atoms are broken down and new bonds are formed. Atoms, however, can be created or destroyed in nuclear reactions: radioactive decays, nuclear fission and fusion. ...
mass of an atom - CHM101-02
... 1 amu = 1.667 x 10-24 g In the periodic table, not all atomic masses (atomic weights) are close to a whole number because of the existence of isotopes. ...
... 1 amu = 1.667 x 10-24 g In the periodic table, not all atomic masses (atomic weights) are close to a whole number because of the existence of isotopes. ...
Ch 11 Atoms etc GNC
... What do you know about hydrogen, based only on the fact that it is a nonmetal? It is probably a gas at room temperature. It probably does not conduct heat and electricity very well. It is probably dull in appearance. It cannot change shape without breaking. It is a basis of the chemicals of life. Se ...
... What do you know about hydrogen, based only on the fact that it is a nonmetal? It is probably a gas at room temperature. It probably does not conduct heat and electricity very well. It is probably dull in appearance. It cannot change shape without breaking. It is a basis of the chemicals of life. Se ...
Gr 10 Review sheet chemistry
... 2. Formation of a ________________ 3. Formation of _____________ 4. Release or absorption of_____________ ...
... 2. Formation of a ________________ 3. Formation of _____________ 4. Release or absorption of_____________ ...