Chapter 2 Matter is Made up of Atoms
... changing, the theory becomes a Scientific Law (Like Gravity) ...
... changing, the theory becomes a Scientific Law (Like Gravity) ...
Atoms have a structure that determines their properties.
... • Metals are found on the left side, non-metals on the right, and metalloids in between. • Chemical families are arranged in vertical groups. • The table can also display the chemical symbol, atomic number, atomic mass, ion charge, density, and other information about each element. ...
... • Metals are found on the left side, non-metals on the right, and metalloids in between. • Chemical families are arranged in vertical groups. • The table can also display the chemical symbol, atomic number, atomic mass, ion charge, density, and other information about each element. ...
Developing Ideas about Matter
... Continuous (no such thing as a smaller piece) Democritus- proposed tiny particles (atomos) Didn’t test their ideas ...
... Continuous (no such thing as a smaller piece) Democritus- proposed tiny particles (atomos) Didn’t test their ideas ...
Atomic Timeline
... ► These orbits are specific distances from the nucleus ► Electron energy level model. ...
... ► These orbits are specific distances from the nucleus ► Electron energy level model. ...
ATOM
... John Dalton (1766-1844) is the father of the chemical atomic theory. He proposed an "atomic theory" with spherical solid atoms based upon measurable properties of mass. The main principles of his theory: chemical elements are made of atoms; atoms of different elements have different masses; atoms of ...
... John Dalton (1766-1844) is the father of the chemical atomic theory. He proposed an "atomic theory" with spherical solid atoms based upon measurable properties of mass. The main principles of his theory: chemical elements are made of atoms; atoms of different elements have different masses; atoms of ...
Atomic Structure and the Composition of Matter
... mass and are ~1800 times more massive than the electron. Both nuclear particles are composed of quarks, smaller fundamental particles. • Protons have unit positive charge (+1), while electrons have unit negative charge (-1). Neutrons ...
... mass and are ~1800 times more massive than the electron. Both nuclear particles are composed of quarks, smaller fundamental particles. • Protons have unit positive charge (+1), while electrons have unit negative charge (-1). Neutrons ...
Midterm Review 2017
... considered in order of increasing atomic number, there is an increase in 1) atomic radius 2) electronegativity 3) first ionization energy 4) number of electrons in the first shell 39) When an atom of phosphorus becomes a phosphide ion (P 3–), the radius ...
... considered in order of increasing atomic number, there is an increase in 1) atomic radius 2) electronegativity 3) first ionization energy 4) number of electrons in the first shell 39) When an atom of phosphorus becomes a phosphide ion (P 3–), the radius ...
Atomic Structure 1
... What happens if... • An atom gains or loses electrons? – you get an ION…a charged particle ...
... What happens if... • An atom gains or loses electrons? – you get an ION…a charged particle ...
Physical Science 1st Semester final Review
... 35.A material that is malleable and conducts electricity well is most likely a __________________________________. ...
... 35.A material that is malleable and conducts electricity well is most likely a __________________________________. ...
example - Royal Society of Chemistry
... - (Limitation) Different substances undergo changes of state at different temperatures. The simple particle model used so far does not yet take into account the differences in attraction (bonds) between different types of particles. Classify substances as elements, mixtures and compounds (e.g., diff ...
... - (Limitation) Different substances undergo changes of state at different temperatures. The simple particle model used so far does not yet take into account the differences in attraction (bonds) between different types of particles. Classify substances as elements, mixtures and compounds (e.g., diff ...
john dalton!! - Hawk Chemistry
... • He was born September 6th, 1766 in Eaglesfield in Cumberland. • He died on July 27th, ...
... • He was born September 6th, 1766 in Eaglesfield in Cumberland. • He died on July 27th, ...
chapter_four
... found outside the nucleus in regions called orbitals Protons are positively charged and found in the nucleus of an atom with neutrons, which have no charge There are even smaller particles but we do not study ...
... found outside the nucleus in regions called orbitals Protons are positively charged and found in the nucleus of an atom with neutrons, which have no charge There are even smaller particles but we do not study ...
PowerPoint Presentation - Introduction to Atoms & Nuclei
... can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. ...
... can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. ...
Element Blocks Project
... Your assignment is to produce an element block that will have six sides, each having different information about your element. Elements will be assigned randomly. Your teacher will show you how to make the block after you have researched and obtained all the information that will go onto you block. ...
... Your assignment is to produce an element block that will have six sides, each having different information about your element. Elements will be assigned randomly. Your teacher will show you how to make the block after you have researched and obtained all the information that will go onto you block. ...
Chapter 8 Study Guide
... b. Atoms of a given element are identical in their physical and chemical properties. c. Atoms of different elements differ in their physical and chemical properties. d. Atoms of different elements combine in simple, whole number ratios to form compounds e. In chemical reactions, atoms are combined, ...
... b. Atoms of a given element are identical in their physical and chemical properties. c. Atoms of different elements differ in their physical and chemical properties. d. Atoms of different elements combine in simple, whole number ratios to form compounds e. In chemical reactions, atoms are combined, ...
BASIC CHEMISTRY
... In our biosphere, everything is made of atoms Through the interaction of chemicals we can better understand our biosphere Give an example from what we have already done in Bio. ...
... In our biosphere, everything is made of atoms Through the interaction of chemicals we can better understand our biosphere Give an example from what we have already done in Bio. ...
WARM UP 9/17
... because of too many or not enough electrons (makes the atom more reactive) ANION – Too many e- , so charge is negative ...
... because of too many or not enough electrons (makes the atom more reactive) ANION – Too many e- , so charge is negative ...
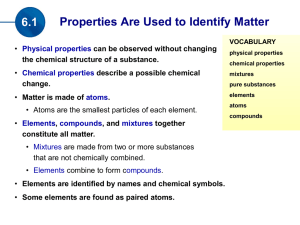
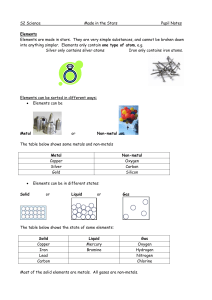
Made in the Stars Notes
... The vertical columns are called Groups: Group 1 Alkali metals Group 2 Alkali earth metals Group 7 Halogens Group 8 (or 0) Noble gases All of the elements in the same group have similar chemical properties, e.g. Group 1 metals are all very reactive, very soft and have to be stored under oil. Group 8 ...
... The vertical columns are called Groups: Group 1 Alkali metals Group 2 Alkali earth metals Group 7 Halogens Group 8 (or 0) Noble gases All of the elements in the same group have similar chemical properties, e.g. Group 1 metals are all very reactive, very soft and have to be stored under oil. Group 8 ...
Chemistry Review- Answer all questions on loose
... f) Silver cutlery tarnishing over time. Chemical change 7. What were the major contributions of the following scientists to the study of chemistry: a) Dalton (include Dalton’s atomic theory) Main points are: ...
... f) Silver cutlery tarnishing over time. Chemical change 7. What were the major contributions of the following scientists to the study of chemistry: a) Dalton (include Dalton’s atomic theory) Main points are: ...