PowerPoint
... • He determined that most of the atom was made up of 'empty space'. • Before this, everyone figured atoms were solid mixes of all the different particles. • This is how we know the weight of an atom is all in a small nucleus in the center. ...
... • He determined that most of the atom was made up of 'empty space'. • Before this, everyone figured atoms were solid mixes of all the different particles. • This is how we know the weight of an atom is all in a small nucleus in the center. ...
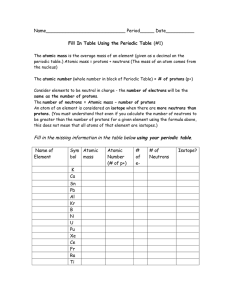
Periodic Table Fill in Table 1
... The atomic mass is the average mass of an element (given as a decimal on the periodic table.) Atomic mass = protons + neutrons (The mass of an atom comes from the nucleus) The atomic number (whole number in block of Periodic Table) = # of protons (p+) Consider elements to be neutral in charge - the ...
... The atomic mass is the average mass of an element (given as a decimal on the periodic table.) Atomic mass = protons + neutrons (The mass of an atom comes from the nucleus) The atomic number (whole number in block of Periodic Table) = # of protons (p+) Consider elements to be neutral in charge - the ...
Periodic Table Jeopardy
... Atomic Theory with evidence. He had four key postulates that he wanted everyone to know. ...
... Atomic Theory with evidence. He had four key postulates that he wanted everyone to know. ...
Notes - Organization of Matter
... • Compounds are pure substances that are composed of two or more atoms that are chemically combined • Compounds can only be changed into simpler substances called elements by chemical changes ...
... • Compounds are pure substances that are composed of two or more atoms that are chemically combined • Compounds can only be changed into simpler substances called elements by chemical changes ...
study guide - atomic srtucture/_classification of matter
... Nuclear symbol: Show the element’s symbol, the atomic mass & the atomic number. Mass goes on the top left of the symbol, number goes on the bottom left of the symbol. mass#atomic number X Bohr Model: Shows number of electrons in each energy level around the nucleus. Mixtures vs. Substances ...
... Nuclear symbol: Show the element’s symbol, the atomic mass & the atomic number. Mass goes on the top left of the symbol, number goes on the bottom left of the symbol. mass#atomic number X Bohr Model: Shows number of electrons in each energy level around the nucleus. Mixtures vs. Substances ...
Atomic Number
... -Elements within the same group have similar properties EX. Au, Ag, Cu -Each horizontal row is called a ____________________ -Properties of the elements gradually change when you move through a period -Elements get smaller when you move from _________________ to ______________. ...
... -Elements within the same group have similar properties EX. Au, Ag, Cu -Each horizontal row is called a ____________________ -Properties of the elements gradually change when you move through a period -Elements get smaller when you move from _________________ to ______________. ...
2 IONS
... Because electrons are on the outside, sometimes electrons can be gained from or lost to another atom. These atoms are called ions. Cations are positive (LOST ELECTRON) and are formed by elements on the left side of the periodic chart. Anions are negative (GAINED ELECTRON) and are formed by eleme ...
... Because electrons are on the outside, sometimes electrons can be gained from or lost to another atom. These atoms are called ions. Cations are positive (LOST ELECTRON) and are formed by elements on the left side of the periodic chart. Anions are negative (GAINED ELECTRON) and are formed by eleme ...
atoms
... elements composed of atoms atoms of the same element are alike different atoms can combine in ratios to form compounds chemical reactions can occur when atoms are separated, joined, or rearranged (but atoms are not created nor destroyed) ...
... elements composed of atoms atoms of the same element are alike different atoms can combine in ratios to form compounds chemical reactions can occur when atoms are separated, joined, or rearranged (but atoms are not created nor destroyed) ...
Reporting Category 1 Answer Key
... How many groups or families of elements are on the table? 18 How many periods of elements are on the table? 7 Valence Electrons – outermost electrons, determine chemical properties and reactivity Elements have similar properties because? They have the same # of valence electrons Reactivity – how eas ...
... How many groups or families of elements are on the table? 18 How many periods of elements are on the table? 7 Valence Electrons – outermost electrons, determine chemical properties and reactivity Elements have similar properties because? They have the same # of valence electrons Reactivity – how eas ...
Reporting Category 1 Answer Key
... How many groups or families of elements are on the table? 18 How many periods of elements are on the table? 7 Valence Electrons – outermost electrons, determine chemical properties and reactivity Elements have similar properties because? They have the same # of valence electrons Reactivity – how eas ...
... How many groups or families of elements are on the table? 18 How many periods of elements are on the table? 7 Valence Electrons – outermost electrons, determine chemical properties and reactivity Elements have similar properties because? They have the same # of valence electrons Reactivity – how eas ...
Atomic structure unit powerpoint
... other. In making such alignments Mendeleev was able to determine that several, as yet unidentified, elements should exist (the elements with masses 44, 68 and 72 are examples). He went on to make predictions about the properties of these missing elements which aided in their discovery. The discovery ...
... other. In making such alignments Mendeleev was able to determine that several, as yet unidentified, elements should exist (the elements with masses 44, 68 and 72 are examples). He went on to make predictions about the properties of these missing elements which aided in their discovery. The discovery ...
History of Chemistry PPT
... • Elements are made of tiny particles called atoms • All atoms of the same element are identical (later to be revised) • The atoms of a given element are different from those of any other element • Elements combine to form compounds • Mass is neither created or destroyed in a chemical reaction, but ...
... • Elements are made of tiny particles called atoms • All atoms of the same element are identical (later to be revised) • The atoms of a given element are different from those of any other element • Elements combine to form compounds • Mass is neither created or destroyed in a chemical reaction, but ...
The Atom Part 1 Notes
... • Smallest particle of an element that retains its identity in a chemical reaction • Democritus, 460 B.C., a philosopher • First to suggest atoms exist • Main thought • Atoms were indivisible and indestructible • Lacked scientific support ...
... • Smallest particle of an element that retains its identity in a chemical reaction • Democritus, 460 B.C., a philosopher • First to suggest atoms exist • Main thought • Atoms were indivisible and indestructible • Lacked scientific support ...
Biochemistry Introduction day 1
... Chemical Reactions: when elements and compounds interact with each other to form new substances. Reactant: A substance that undergoes a chemical reaction. Product: A substance formed from chemical reaction. Chemical Equations: Communicate what is happening in a chemical reaction. It can be done in a ...
... Chemical Reactions: when elements and compounds interact with each other to form new substances. Reactant: A substance that undergoes a chemical reaction. Product: A substance formed from chemical reaction. Chemical Equations: Communicate what is happening in a chemical reaction. It can be done in a ...
CHAPTER 1 Practice Exercises 1.1 12.3 g Cd 1.3 26.9814 u 1.5
... Silver and gold are in the same periodic table group as copper, so they might well be expected to occur together in nature, because of their similar properties and tendencies to form similar compounds. ...
... Silver and gold are in the same periodic table group as copper, so they might well be expected to occur together in nature, because of their similar properties and tendencies to form similar compounds. ...
Section 12.4 - CPO Science
... ingredient of DNA, the molecule responsible for carrying the genetic code in all living creatures. When phosphorus atoms absorb light, they store energy, then release it in a greenish glow. ...
... ingredient of DNA, the molecule responsible for carrying the genetic code in all living creatures. When phosphorus atoms absorb light, they store energy, then release it in a greenish glow. ...
More Chemistry!
... useful way The first Periodic Table of Elements was proposed by a Russian – Dmitri Mendeleev in ...
... useful way The first Periodic Table of Elements was proposed by a Russian – Dmitri Mendeleev in ...
matter crct/final exam review
... 41. Why do atoms share valence electrons or transfer valence electrons? 42. What is the difference between a compound and an element? ...
... 41. Why do atoms share valence electrons or transfer valence electrons? 42. What is the difference between a compound and an element? ...
Topic 3 – Atoms and the Periodic Table – Learning Outcomes
... us the electron arrangement for all the elements. We are just interested in the first 20 in standard grade and we can use this information on page 1 to draw target diagrams for the first 20 elements. Elements in the same group have the same number of outer electrons e.g. lithium, sodium, potassium e ...
... us the electron arrangement for all the elements. We are just interested in the first 20 in standard grade and we can use this information on page 1 to draw target diagrams for the first 20 elements. Elements in the same group have the same number of outer electrons e.g. lithium, sodium, potassium e ...
Atoms and the Periodic Table Study Guide
... 2) Complete the table below then use the diagram to compare and contrast the subatomic particles. Protons ...
... 2) Complete the table below then use the diagram to compare and contrast the subatomic particles. Protons ...
introductory chemistry
... Elements are substances that contain only one type of atom. Hydrogen gas is an element as it contains only hydrogen atoms. Compounds contain the atoms of two or more different elements joined together. Water is a compound that consists of hydrogen and oxygen atoms joined together. There are nearly 1 ...
... Elements are substances that contain only one type of atom. Hydrogen gas is an element as it contains only hydrogen atoms. Compounds contain the atoms of two or more different elements joined together. Water is a compound that consists of hydrogen and oxygen atoms joined together. There are nearly 1 ...
Midterm Review.ppt - Chemistry R: 4(AE)
... • Which set of elements contains a metalloid? 1. K, Mn, As, Ar 2. Li, Mg, Ca, Kr 3. Ba, Ag, Sn, Xe 4. Fr, F, O, Rn ...
... • Which set of elements contains a metalloid? 1. K, Mn, As, Ar 2. Li, Mg, Ca, Kr 3. Ba, Ag, Sn, Xe 4. Fr, F, O, Rn ...
Atomic Models 2015-2016
... • To make molecules, you must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
... • To make molecules, you must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...