History of the Atom and Periodic Table
... had a neutral charge and it is called the neutron. His discovery made us realize isotopes existed. Isotopes are atoms of the same element with a different number of neutrons. Proved Dalton’s Atomic theory was incorrect again by showing atoms of the same element can be different. ...
... had a neutral charge and it is called the neutron. His discovery made us realize isotopes existed. Isotopes are atoms of the same element with a different number of neutrons. Proved Dalton’s Atomic theory was incorrect again by showing atoms of the same element can be different. ...
Chem 400 Chem 150 REVIEW SHEET Amanda R
... Draw a Lewis structure for any molecule and find bond order ...
... Draw a Lewis structure for any molecule and find bond order ...
September 28th Notes
... Atomic Structure Element: matter that is composed of one type of atom. Elements are abbreviated in scientific shorthand- either a letter or a pair of letters called a chemical symbol. Ex- Aluminum =Al Copper=Cu Atom- smallest piece of matter that still has the properties of the element. Protons- pos ...
... Atomic Structure Element: matter that is composed of one type of atom. Elements are abbreviated in scientific shorthand- either a letter or a pair of letters called a chemical symbol. Ex- Aluminum =Al Copper=Cu Atom- smallest piece of matter that still has the properties of the element. Protons- pos ...
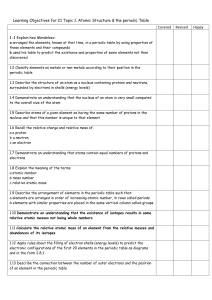
Learning Objectives
... of atomic structure. 4. Distinguish between each of the following pairs of terms: a. neutron and proton b. atomic number and mass number c. atomic weight and mass number 5. Explain how the atomic number and mass number of an atom can be used to determine the number of neutrons. 6. Explain how two is ...
... of atomic structure. 4. Distinguish between each of the following pairs of terms: a. neutron and proton b. atomic number and mass number c. atomic weight and mass number 5. Explain how the atomic number and mass number of an atom can be used to determine the number of neutrons. 6. Explain how two is ...
Power point on the Periodic Table
... Neutron: in the nucleus, symbol “n”, no charge, slightly larger mass than a proton ...
... Neutron: in the nucleus, symbol “n”, no charge, slightly larger mass than a proton ...
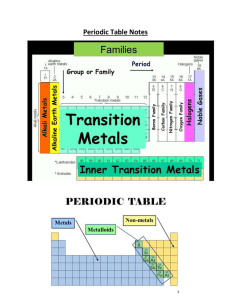
Period Table, valence Electrons and Ion Notes
... Example: Na = 1s2 2s2 2p6 3s1 Add up the e-‘s found in the last energy level, in this case there is only 1 so Na has 1 valence e**You have to do this for the Transition metal every time** ...
... Example: Na = 1s2 2s2 2p6 3s1 Add up the e-‘s found in the last energy level, in this case there is only 1 so Na has 1 valence e**You have to do this for the Transition metal every time** ...
Chemistry 102B What`s in an atom? Before “Chemistry” Other Early
... • Alchemy/Alchemists - a pseudoscience built around trying to turn cheap metals into GOLD! (400 B.C.-1400 A.D.) • Metallurgy – systematic extraction of metals from ores laid some groundwork for modern chemistry. (1500s) • The first “chemist” was Robert Boyle who worked on pressure and volume of gase ...
... • Alchemy/Alchemists - a pseudoscience built around trying to turn cheap metals into GOLD! (400 B.C.-1400 A.D.) • Metallurgy – systematic extraction of metals from ores laid some groundwork for modern chemistry. (1500s) • The first “chemist” was Robert Boyle who worked on pressure and volume of gase ...
Chemistry Overview
... Elements • Element: pure substance that can’t be broken down into simpler substances • Element Symbols – 1-3 letters – Begin with 1 capital letter – Some based on Latin names • Ex/ gold = Au for Aurum iron = Fe for Ferros ...
... Elements • Element: pure substance that can’t be broken down into simpler substances • Element Symbols – 1-3 letters – Begin with 1 capital letter – Some based on Latin names • Ex/ gold = Au for Aurum iron = Fe for Ferros ...
C2- Topic 1: Atomic structure and the periodic table. Assessable
... Explain how Mendeleev: - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
... Explain how Mendeleev: - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
C2 Topic 1 Can Do Sheet
... 1.1 Explain how Mendeleev: a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according ...
... 1.1 Explain how Mendeleev: a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according ...
Quiz review
... Horizontal rows of the periodic table are called this. Vertical columns of the periodic table are called ‘groups’ or this. Which element in period 3 has 6 valence electrons? Which element in period 5 has only 1 electron in its 5s sublevel? Which element in period 3 has a full octet? What family of e ...
... Horizontal rows of the periodic table are called this. Vertical columns of the periodic table are called ‘groups’ or this. Which element in period 3 has 6 valence electrons? Which element in period 5 has only 1 electron in its 5s sublevel? Which element in period 3 has a full octet? What family of e ...
NOTES: 2.1 - Intro to Chemistry
... Isotopes: atoms of an element that have different # of neutrons ● in nature, elements occur as mixtures of isotopes ● some are radioactive: unstable isotope where nucleus decays emitting subatomic particles and/or energy as radioactivity causing one element to transform into another element ...
... Isotopes: atoms of an element that have different # of neutrons ● in nature, elements occur as mixtures of isotopes ● some are radioactive: unstable isotope where nucleus decays emitting subatomic particles and/or energy as radioactivity causing one element to transform into another element ...
Chapter 6 Review“The Periodic Table”
... 11. How do the isotopes of hydrogen-1 and hydrogen-2 differ? 12. How many protons are present in an atom of Beryllium-9? 13. Calculate the number of neutrons in Lead-210. 14. Determine the number of electrons in an atom of Iridium. 15. About how many more times massive is a proton than electron? 16. ...
... 11. How do the isotopes of hydrogen-1 and hydrogen-2 differ? 12. How many protons are present in an atom of Beryllium-9? 13. Calculate the number of neutrons in Lead-210. 14. Determine the number of electrons in an atom of Iridium. 15. About how many more times massive is a proton than electron? 16. ...
File
... nucleus of an atom. (The number of protons in the nucleus is the atomic number, which determines the identity of an element.) neutron: a subatomic particle that has no charge and that is located in the nucleus of an atom. electron: a subatomic particle that has a negative charge. element: a substanc ...
... nucleus of an atom. (The number of protons in the nucleus is the atomic number, which determines the identity of an element.) neutron: a subatomic particle that has no charge and that is located in the nucleus of an atom. electron: a subatomic particle that has a negative charge. element: a substanc ...
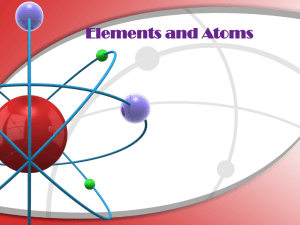
Elements and Atoms - Portola Middle School
... neutron or proton. Protons should have a + or P written on them. Neutrons should be blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
... neutron or proton. Protons should have a + or P written on them. Neutrons should be blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
Summative Assessment Study Guide Name: Due date: SPS1
... Summative Assessment Study Guide Name: __________________________________Due date: _____________________ SPS1. Students will investigate our current understanding of the atom. a. Examine the structure of the atom in terms of ...
... Summative Assessment Study Guide Name: __________________________________Due date: _____________________ SPS1. Students will investigate our current understanding of the atom. a. Examine the structure of the atom in terms of ...