ch2_objectives
... 4. Draw and label a simplified model of an atom. Explain how this model simplifies our understanding of atomic structure. 5. Distinguish between each of the following pairs of terms: a. neutron and proton b. atomic number and mass number c. atomic weight and mass number 6. Explain how the atomic num ...
... 4. Draw and label a simplified model of an atom. Explain how this model simplifies our understanding of atomic structure. 5. Distinguish between each of the following pairs of terms: a. neutron and proton b. atomic number and mass number c. atomic weight and mass number 6. Explain how the atomic num ...
Periodic Table
... Dalton’s Atomic Theory 1. All matter is composed of extremely small particles ...
... Dalton’s Atomic Theory 1. All matter is composed of extremely small particles ...
17 review for test - Blair Community Schools
... • who came up with the Plum Pudding model? • who discovered the nucleus? • who came up with the word “atom” meaning • Who put electrons in orbits? indivsible Completion (10) These terms will be used for the completion • Metals • Metalloids • Mass number • Isotopes • Transition elements • Periods • A ...
... • who came up with the Plum Pudding model? • who discovered the nucleus? • who came up with the word “atom” meaning • Who put electrons in orbits? indivsible Completion (10) These terms will be used for the completion • Metals • Metalloids • Mass number • Isotopes • Transition elements • Periods • A ...
Bill Nye Atoms Workseet
... The particle in the middle are very (circle one) heavy light Buzzing around the outside the particles are very (circle one) heavy light In Bill’s model, how far away is the electron from the nucleus? ______________________________________ If atoms are mostly empty space, how come they act like a sol ...
... The particle in the middle are very (circle one) heavy light Buzzing around the outside the particles are very (circle one) heavy light In Bill’s model, how far away is the electron from the nucleus? ______________________________________ If atoms are mostly empty space, how come they act like a sol ...
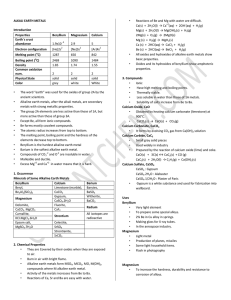
ALKALI EARTH METALS Introduction Properties Beryllium
... The word “earth” was used for the oxides of group 2A by the ancient scientists. Alkaline earth metals, after the alkali metals, are secondary metals with strong metallic properties. The group 2A elements are less active than those of 1A, but more active than those of group 3A. Except Be, all form io ...
... The word “earth” was used for the oxides of group 2A by the ancient scientists. Alkaline earth metals, after the alkali metals, are secondary metals with strong metallic properties. The group 2A elements are less active than those of 1A, but more active than those of group 3A. Except Be, all form io ...
Review 1st Qtr KEY
... PART I: You should know the basics (terms & basic facts) about… Hypothesis theory law scientific method mass weight volume physical change chemical change mixture element compound molecule heterogeneous homogeneous states of matter ...
... PART I: You should know the basics (terms & basic facts) about… Hypothesis theory law scientific method mass weight volume physical change chemical change mixture element compound molecule heterogeneous homogeneous states of matter ...
Physical Science Chapter 6 Study Guide Atomic Theory of Matter
... Nuclear reactor—a type of controlled reaction used to harness useful energy ...
... Nuclear reactor—a type of controlled reaction used to harness useful energy ...
Nuclear radiation 4
... output of the reactor is 1·4GW. How many fission reactions are produced in one hour? ...
... output of the reactor is 1·4GW. How many fission reactions are produced in one hour? ...
Matter and the Periodic Table Study Guide Answer Key
... Semimetals/Metalloids have properties of both metals and non-metals. 3.b. Compounds are formed by combining two or more different elements and compounds have properties that are different from their constituent elements. 3.f. Use the periodic table to identify elements in simple compounds. Compound ...
... Semimetals/Metalloids have properties of both metals and non-metals. 3.b. Compounds are formed by combining two or more different elements and compounds have properties that are different from their constituent elements. 3.f. Use the periodic table to identify elements in simple compounds. Compound ...
atomic number - Thomas C. Cario Middle School
... The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of atom. The atomic number of an atom is equal to the number of protons in its nucleus. The number of electrons surrounding the nucleus of an atom is equal ...
... The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of atom. The atomic number of an atom is equal to the number of protons in its nucleus. The number of electrons surrounding the nucleus of an atom is equal ...
What is Matter
... Smallest part or piece of matter that still has the properties of matter Proton: Positively charged particle; largest of three particles; symbol is P+ Neutron: Neutrally charged particle; has both positive and negative charges; slightly smaller than proton; symbol is N= Electron: Negatively charge p ...
... Smallest part or piece of matter that still has the properties of matter Proton: Positively charged particle; largest of three particles; symbol is P+ Neutron: Neutrally charged particle; has both positive and negative charges; slightly smaller than proton; symbol is N= Electron: Negatively charge p ...
here
... After reading these sections complete two of the following three options: 1. Explain what a valence electron is, and why it might make sense for valence electrons to be the “bonding/reactive” electrons. Then give an example of an element that would be likely to bond and one that would not be likely ...
... After reading these sections complete two of the following three options: 1. Explain what a valence electron is, and why it might make sense for valence electrons to be the “bonding/reactive” electrons. Then give an example of an element that would be likely to bond and one that would not be likely ...
Chemical reactions revision
... A name ending in ‘...ide’ means that the compound contains two elements A name ending in ‘...ate’ means that the compound contains three elements and one is oxygen. ‘Oxygen’ does not show up in the name; the ‘ate’ is the only clue it is there You should be able to give the name of the compound forme ...
... A name ending in ‘...ide’ means that the compound contains two elements A name ending in ‘...ate’ means that the compound contains three elements and one is oxygen. ‘Oxygen’ does not show up in the name; the ‘ate’ is the only clue it is there You should be able to give the name of the compound forme ...
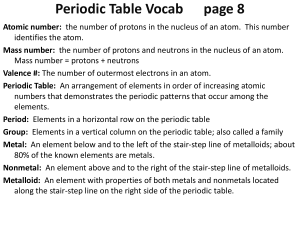
Periodic Table Vocab page 7
... Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elements in order of increasing atomic numbers that demonstrates the periodic patterns that occur amo ...
... Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elements in order of increasing atomic numbers that demonstrates the periodic patterns that occur amo ...
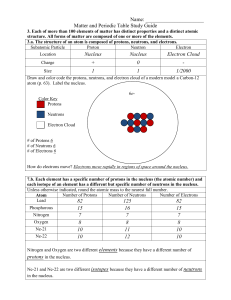
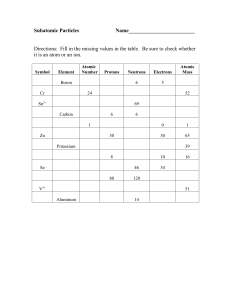
Understanding the Atom GN
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
Atom - Sites
... atoms join together chemically. •Combinations of two or more different elements are called compounds. •All compounds are molecules but not all molecules are compounds. (ex. H2O vs. O2) •Molecules can also join together to form larger molecules. •Many, many repeating small molecules joined together f ...
... atoms join together chemically. •Combinations of two or more different elements are called compounds. •All compounds are molecules but not all molecules are compounds. (ex. H2O vs. O2) •Molecules can also join together to form larger molecules. •Many, many repeating small molecules joined together f ...
Chemistry Review: Antoine Lavoisier (1743
... there are 3 isotopes of hydrogen, with mass numbers of 1, 2 and 3. These atoms have different masses because they have different numbers of neutrons. However, since they have the same number of protons, they are the same element. Most elements have more than 1 naturally occurring isotope. However, t ...
... there are 3 isotopes of hydrogen, with mass numbers of 1, 2 and 3. These atoms have different masses because they have different numbers of neutrons. However, since they have the same number of protons, they are the same element. Most elements have more than 1 naturally occurring isotope. However, t ...
CLASS TEST NAME Class IIB Date ______ 1 .Which atomic
... 21. The electrons ______________________________________________________ around the nucleus in shells. The first shell, which is _______________________________ the nucleus, can hold ________electrons, whereas the 2nd and 3rd shells can hold ...
... 21. The electrons ______________________________________________________ around the nucleus in shells. The first shell, which is _______________________________ the nucleus, can hold ________electrons, whereas the 2nd and 3rd shells can hold ...
Chapter 7 Review Sheet
... creature approaches you. He explains that he is a scientist from planet Rainhard in a far corner of the universe. Only 12 elements are known to exist on Rainhard. He has been trying unsuccessfully to organize a periodic table for these elements, and in desperation has come to the far-off planet Eart ...
... creature approaches you. He explains that he is a scientist from planet Rainhard in a far corner of the universe. Only 12 elements are known to exist on Rainhard. He has been trying unsuccessfully to organize a periodic table for these elements, and in desperation has come to the far-off planet Eart ...
The Chemical Basis of Life Chapter 4
... •Made of only one type of atom •Represented by 1-2 letters ...
... •Made of only one type of atom •Represented by 1-2 letters ...