Chapter 6 Vocabulary crossword puzzle
... 3. Elements in which the highest occupied s and p sublevels are partially filled 6. Measures the ability of an atom to attract electrons when the atom is in a compound; the element named Cesium has the lowest amount, while the element named Fluorine has the highest amount 7. Term that refers to a se ...
... 3. Elements in which the highest occupied s and p sublevels are partially filled 6. Measures the ability of an atom to attract electrons when the atom is in a compound; the element named Cesium has the lowest amount, while the element named Fluorine has the highest amount 7. Term that refers to a se ...
Notes
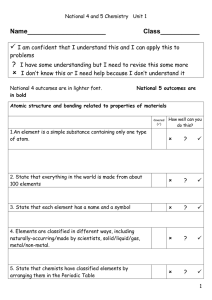
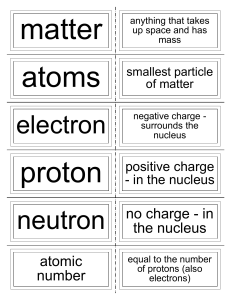
... -the number of protons in an atom of an element •all atoms of an element have the same atomic # •written as a subscript next to the element’s symbol •in a neutral atom, the number of protons is equal to the number of electrons (balanced charges). ...
... -the number of protons in an atom of an element •all atoms of an element have the same atomic # •written as a subscript next to the element’s symbol •in a neutral atom, the number of protons is equal to the number of electrons (balanced charges). ...
L.O.
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
Introduction_to_Geochemistry_Pre-Lecture_Quiz
... detach the loosest electron from atoms of that element. (e) All alkali metals have similar chemical properties. (f) Alkali earths have one electron in the outer shell. (g) Electronegativity is the amount of negative charge on an atom. (h) Ca has a valency of 2. (i) True ionic bonds are unknown and a ...
... detach the loosest electron from atoms of that element. (e) All alkali metals have similar chemical properties. (f) Alkali earths have one electron in the outer shell. (g) Electronegativity is the amount of negative charge on an atom. (h) Ca has a valency of 2. (i) True ionic bonds are unknown and a ...
Thursday, October 31, 2013 D-day
... – Most reactive nonmetals. – These elements are all brightly colored. ...
... – Most reactive nonmetals. – These elements are all brightly colored. ...
2.1 The Nature of Matter - Sonoma Valley High School
... Some elements have isotopes, with different #s of neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
... Some elements have isotopes, with different #s of neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
Atomic Crossword Name: Period: ____
... 11. A _____________ electron is in the outermost shell of an atom 12. Formed when atoms share electrons 14. Has a different number of neutrons than normal 15. The splitting of an atomic nucleus (usually with heavy elements) 17. British scientist who formulated our modern atomic theory 22. The mergin ...
... 11. A _____________ electron is in the outermost shell of an atom 12. Formed when atoms share electrons 14. Has a different number of neutrons than normal 15. The splitting of an atomic nucleus (usually with heavy elements) 17. British scientist who formulated our modern atomic theory 22. The mergin ...
Homework Geochem Test Review
... 2. What is smallest part of an element that has all the properties of that element? ...
... 2. What is smallest part of an element that has all the properties of that element? ...
Chapter 5
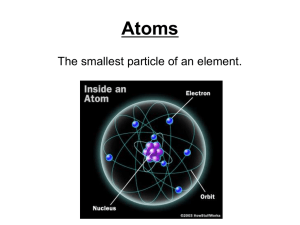
... •Electron cloud is 10,000 times larger than the nucleus, but is still mostly empty. •Electrons are in the cloud but can not be pinpointed at an exact time because they move so quickly. ...
... •Electron cloud is 10,000 times larger than the nucleus, but is still mostly empty. •Electrons are in the cloud but can not be pinpointed at an exact time because they move so quickly. ...
IPC Atoms and Periodic Table
... of the naturally occurring isotopes of an element • Reported as atomic mass on the periodic ...
... of the naturally occurring isotopes of an element • Reported as atomic mass on the periodic ...
Learning Standards vocab chemical basis and molecules of life 09
... sources of food and nutrition, fossil fuels). Describe at least three chemical reactions of particular importance to humans (e.g., burning of fossil fuels, photosynthesis, rusting of metals). Use a chemical equation to illustrate how the atoms in molecules are arranged before and after a reactio ...
... sources of food and nutrition, fossil fuels). Describe at least three chemical reactions of particular importance to humans (e.g., burning of fossil fuels, photosynthesis, rusting of metals). Use a chemical equation to illustrate how the atoms in molecules are arranged before and after a reactio ...
CHEMISTRY TERMS Period: Elements in the same horizontal row
... Period: Elements in the same horizontal row with the same ground state energy level. Periodic Law: Elements list in order of their atomic numbers that fall into reoccurring groups. Ionic Radius: the radius of an atom’s ion, measured by the distance between ions in a crystal lattice. Atomic Radius: o ...
... Period: Elements in the same horizontal row with the same ground state energy level. Periodic Law: Elements list in order of their atomic numbers that fall into reoccurring groups. Ionic Radius: the radius of an atom’s ion, measured by the distance between ions in a crystal lattice. Atomic Radius: o ...
Chemical Basis of Life
... Title: The Chemical Basis of Life 1- Introduction: Your body is an elaborate chemical system. Chemical reactions power all of the body’s activities. At the most basic level, life is about chemicals and how they interact with each other. 2- Matter – Matter is anything that has mass and occupies space ...
... Title: The Chemical Basis of Life 1- Introduction: Your body is an elaborate chemical system. Chemical reactions power all of the body’s activities. At the most basic level, life is about chemicals and how they interact with each other. 2- Matter – Matter is anything that has mass and occupies space ...