1000 - Paint Valley Local Schools
... metals have only one valence electron in their outer shell. Therefore, they are ready to lose that one electron in ionic bonding with other elements very easily. This makes them _______ _________ especially with group 17 because they have 7 valence electrons and when the two combine they make the ma ...
... metals have only one valence electron in their outer shell. Therefore, they are ready to lose that one electron in ionic bonding with other elements very easily. This makes them _______ _________ especially with group 17 because they have 7 valence electrons and when the two combine they make the ma ...
Periodic Trends
... the periodic table • Radius of the atom – Distance between the nucleus and outermost electrons ...
... the periodic table • Radius of the atom – Distance between the nucleus and outermost electrons ...
1.2 Atomic Theory
... The average atomic mass for magnesium found on the periodic table is a weighted average of the three isotopes: 24.31 g of Mg Radioactivity: spontaneous decay of nuclei, releasing energy and subatomic particles Radioisotopes: an unstable isotope of an element, which undergoes radioactive decay ...
... The average atomic mass for magnesium found on the periodic table is a weighted average of the three isotopes: 24.31 g of Mg Radioactivity: spontaneous decay of nuclei, releasing energy and subatomic particles Radioisotopes: an unstable isotope of an element, which undergoes radioactive decay ...
Unit Description - Honors Chemistry
... Chapters 1 and 3 – Scientific Method and Matter Distinguish among hypothesis, theory and scientific law using examples. Identify the common steps of scientific methods. Distinguish between qualitative and quantitative data. Distinguish between independent and dependent variables, controls an ...
... Chapters 1 and 3 – Scientific Method and Matter Distinguish among hypothesis, theory and scientific law using examples. Identify the common steps of scientific methods. Distinguish between qualitative and quantitative data. Distinguish between independent and dependent variables, controls an ...
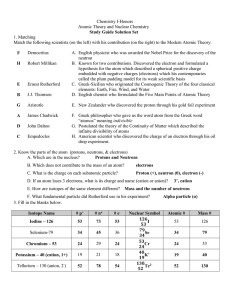
Name Period ______ Unit 4 Study Guide A common isotope of iron
... The lowest energy level can hold ________ electrons. A repeating pattern across a row of the periodic table is called…. The noble gases such as helium and xenon do not form chemical bonds with other elements because they: Most metals are ________________ (choose one: ductile, brittle, poor conductor ...
... The lowest energy level can hold ________ electrons. A repeating pattern across a row of the periodic table is called…. The noble gases such as helium and xenon do not form chemical bonds with other elements because they: Most metals are ________________ (choose one: ductile, brittle, poor conductor ...
Chapter 4 notes outline
... number of neutrons Elements can have several isotopes 4.3 Modern Atomic Theory Bohr’s Model of the Atom Better description of electrons Electrons orbit around nucleus in energy levels like planets 1st Level = holds up to 2 electrons 2nd Level = holds up to 8 electrons Electrons can move to d ...
... number of neutrons Elements can have several isotopes 4.3 Modern Atomic Theory Bohr’s Model of the Atom Better description of electrons Electrons orbit around nucleus in energy levels like planets 1st Level = holds up to 2 electrons 2nd Level = holds up to 8 electrons Electrons can move to d ...
Unit 2: Atomic Structure and Nuclear Chemistry
... In this unit students will describe how the arrangement of elements in the periodic table and their electron configurations are related. They will describe how the location of an element in the periodic table can be used to predict the properties of that element. Expected learning outcomes: 1. Deter ...
... In this unit students will describe how the arrangement of elements in the periodic table and their electron configurations are related. They will describe how the location of an element in the periodic table can be used to predict the properties of that element. Expected learning outcomes: 1. Deter ...
Atoms and Molecules
... • Elements are determined by the number of protons in the nucleus of the atom ...
... • Elements are determined by the number of protons in the nucleus of the atom ...
SB Vocab list Word document
... A negatively charged particle found in the shells of an atom. Its mass is approximately 1/2000 that of a proton or neutron. Group A vertical column in the Periodic Table. Elements in the same group have similar chemical properties since they all have the same number of electrons in their outer (or v ...
... A negatively charged particle found in the shells of an atom. Its mass is approximately 1/2000 that of a proton or neutron. Group A vertical column in the Periodic Table. Elements in the same group have similar chemical properties since they all have the same number of electrons in their outer (or v ...
section_2_review_set
... 1. What is the claim to fame for the proton? determines the element 2. What is the claim to fame for the electron? creates the chemical bonds 3. What is the claim to fame for the neutron? stabilizes the nucleus 4. What is the mass of each of the following particles?: proton 1; neutron 1; electron 0. ...
... 1. What is the claim to fame for the proton? determines the element 2. What is the claim to fame for the electron? creates the chemical bonds 3. What is the claim to fame for the neutron? stabilizes the nucleus 4. What is the mass of each of the following particles?: proton 1; neutron 1; electron 0. ...
34.) Write out the set of four quantum numbers for the last electron
... 31.) Write electron configuration for nickel and calcium. 32.) Draw orbital notation for H.O.E.L. of nickel and calcium. 33.) Write noble gas configuration for nickel and calcium. 34.) Write out the set of four quantum numbers for the last electron added to nickel and calcium. (Honors only) 35.) Dra ...
... 31.) Write electron configuration for nickel and calcium. 32.) Draw orbital notation for H.O.E.L. of nickel and calcium. 33.) Write noble gas configuration for nickel and calcium. 34.) Write out the set of four quantum numbers for the last electron added to nickel and calcium. (Honors only) 35.) Dra ...
Study Guide - Honors Chemistry
... one nucleus is broken into multiple (2 in this case) nuclei by force (an alpha particle is used to break it up) one nucleus is broken into multiple (2 in this case) nuclei on its own. No force is needed. one nucleus is transformed into another nucleus by bombarding a particle into it. A particle may ...
... one nucleus is broken into multiple (2 in this case) nuclei by force (an alpha particle is used to break it up) one nucleus is broken into multiple (2 in this case) nuclei on its own. No force is needed. one nucleus is transformed into another nucleus by bombarding a particle into it. A particle may ...
Chapter 3 Atoms and Elements
... All light, whether radiowaves or visible light, travels as the same speed, 3 *108 meters/sec As a result, since the length of each wave decreases from left to right, the frequency of the peaks an troughs of the waves shown above must increase from left to right Referring to light as a particle, know ...
... All light, whether radiowaves or visible light, travels as the same speed, 3 *108 meters/sec As a result, since the length of each wave decreases from left to right, the frequency of the peaks an troughs of the waves shown above must increase from left to right Referring to light as a particle, know ...
unit plan template
... Identify the names and symbols of common elements. Identify quarks as subatomic particles of matter. Describe the electron cloud model of the atom. Explain how electrons are arranged in an atom. Compute the atomic mass and mass number of an atom. Identify the components of isotopes. In ...
... Identify the names and symbols of common elements. Identify quarks as subatomic particles of matter. Describe the electron cloud model of the atom. Explain how electrons are arranged in an atom. Compute the atomic mass and mass number of an atom. Identify the components of isotopes. In ...
File
... Discovered protons in 1900; anode ray experiment; said that since atoms contain negatively charged particles, they must also contain positively charged particles in order to remain electrically neutral ...
... Discovered protons in 1900; anode ray experiment; said that since atoms contain negatively charged particles, they must also contain positively charged particles in order to remain electrically neutral ...
Unit 2 Overview
... An Introduction to the inner workings of the atom, it history, and its structure Objective: To gain a deeper understanding of the role that each of the sub-atomic particles plays in controlling chemical behavior. In this unit, we will seek to learn how the three sub-atomic particles control outward ...
... An Introduction to the inner workings of the atom, it history, and its structure Objective: To gain a deeper understanding of the role that each of the sub-atomic particles plays in controlling chemical behavior. In this unit, we will seek to learn how the three sub-atomic particles control outward ...
CHEMISTRY 1 FINAL EXAM REVIEW
... 2.) Write the formulas and charges for the following ions: Phosphate, nitrite, nitrate, hydroxide, carbonate, ammonium. 3.) What is the total number of atoms in one molecule of C6Hl2O6? 4.) What types of elements when combined would be most likely to form an ionic compound? 5.) What is the ionic cha ...
... 2.) Write the formulas and charges for the following ions: Phosphate, nitrite, nitrate, hydroxide, carbonate, ammonium. 3.) What is the total number of atoms in one molecule of C6Hl2O6? 4.) What types of elements when combined would be most likely to form an ionic compound? 5.) What is the ionic cha ...
Periodic Table Review Key
... 1. Which element has the smallest atomic number? F 2. Which element has the largest atomic number? H 3. Which non-metal has the smallest atomic mass? F 4. Which metal has the largest atomic mass? D 5. Which elements are considered good conductors? C,E,D, 6. Which element is considered semi-conductor ...
... 1. Which element has the smallest atomic number? F 2. Which element has the largest atomic number? H 3. Which non-metal has the smallest atomic mass? F 4. Which metal has the largest atomic mass? D 5. Which elements are considered good conductors? C,E,D, 6. Which element is considered semi-conductor ...
Week 1 Grade 7 Thursday
... Atomic number = number of protons Atomic number = number of electrons in a neutral atom (not an ion) Atomic weight - atomic number = number of neutrons Isotopes have different numbers of neutrons, H normally has 0 neutrons ...
... Atomic number = number of protons Atomic number = number of electrons in a neutral atom (not an ion) Atomic weight - atomic number = number of neutrons Isotopes have different numbers of neutrons, H normally has 0 neutrons ...
Radioisotopes
... • Isotopes are any of the different types of atoms (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have di ...
... • Isotopes are any of the different types of atoms (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have di ...
Deconstructed HS-PS1-2
... could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.] [Assessment Boundary: Assessment is limited to chemical reactions involving main group elements and combustion reactions.] ...
... could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.] [Assessment Boundary: Assessment is limited to chemical reactions involving main group elements and combustion reactions.] ...
GLOSSARY OF SCIENTIFIC TERMS IN THE MYSTERY OF MATTER
... When applied to nuclear reactions, a process in which the splitting of atoms by neutrons releases more neutrons that, in turn, causes more atoms to split. ...
... When applied to nuclear reactions, a process in which the splitting of atoms by neutrons releases more neutrons that, in turn, causes more atoms to split. ...
Atoms and Elements
... Electrons circle the nucleus in a paths called orbits or energy levels. Low-energy = orbit close to nucleus High-energy = orbit father away. Most of an atoms mass is in the nucleus; protons and neutrons have the same mass; electrons as about 1/2000 of a proton ...
... Electrons circle the nucleus in a paths called orbits or energy levels. Low-energy = orbit close to nucleus High-energy = orbit father away. Most of an atoms mass is in the nucleus; protons and neutrons have the same mass; electrons as about 1/2000 of a proton ...