TERM 2 Unit 3 YR 9 SCI It is elementary
... understandings of atomic structure. Students model an atom according to currently accepted understandings. They will identify patterns in atomic structure that allow prediction of the products of chemical reactions and are reflected by the periodic table. They recognise that new substances are forme ...
... understandings of atomic structure. Students model an atom according to currently accepted understandings. They will identify patterns in atomic structure that allow prediction of the products of chemical reactions and are reflected by the periodic table. They recognise that new substances are forme ...
Matter and the Periodic Table
... Groups: Also known as families, the 18 vertical rows The elements in each group have similar characteristics. ...
... Groups: Also known as families, the 18 vertical rows The elements in each group have similar characteristics. ...
14 more about the atomic number
... The atomic number is important it gives the number of protons in an atom of an element. Since atoms are neutral and the charge on an electron is equal and opposite to the charge on a proton, the atomic number also gives the number of electrons in an atom. An element can be defined as a collection of ...
... The atomic number is important it gives the number of protons in an atom of an element. Since atoms are neutral and the charge on an electron is equal and opposite to the charge on a proton, the atomic number also gives the number of electrons in an atom. An element can be defined as a collection of ...
atom - Images
... the electrons in energy levels (the “solar system” model). Bohr was one of the founders of quantum physics – a discipline that states that energy can be given off in small packets or quanta of specific size. Energy levels closer to the nucleus were lower in energy than those farther away. When a spe ...
... the electrons in energy levels (the “solar system” model). Bohr was one of the founders of quantum physics – a discipline that states that energy can be given off in small packets or quanta of specific size. Energy levels closer to the nucleus were lower in energy than those farther away. When a spe ...
answers_to_questions_on_pages_100
... Answers to Questions on Pages 100-101 5. A) Protons and neutrons are found in the nucleus while the electrons orbit the nucleus in their respective energy levels. B) The protons and neutrons make up the majority of the mass of an atom. C) CORRECTION – The electrons make up most of the space as their ...
... Answers to Questions on Pages 100-101 5. A) Protons and neutrons are found in the nucleus while the electrons orbit the nucleus in their respective energy levels. B) The protons and neutrons make up the majority of the mass of an atom. C) CORRECTION – The electrons make up most of the space as their ...
Cycle 4 Topic 1 C1 Atomic Structure Cycle Sheet
... Groups 1 and 7, and other elements in this specification. I can write word equations for the reactions in this specification. ...
... Groups 1 and 7, and other elements in this specification. I can write word equations for the reactions in this specification. ...
Review Questions
... CaCl2 is used as a de-icer on roads in the winter. If it has a density of 2.50 g/cm3, what is the mass of 1250 cm3 block of CaCl2? ...
... CaCl2 is used as a de-icer on roads in the winter. If it has a density of 2.50 g/cm3, what is the mass of 1250 cm3 block of CaCl2? ...
Atomic Structure Power Point
... is a form of an element that has the same number of protons, but different numbers of neutrons. The atomic mass on the periodic table reflects the average mass of all of the known isotopes of an element. Each isotope may have different characteristics. ...
... is a form of an element that has the same number of protons, but different numbers of neutrons. The atomic mass on the periodic table reflects the average mass of all of the known isotopes of an element. Each isotope may have different characteristics. ...
MID-TERM EXAM REVIEW! Unit 1 Convert the following: 1.) 2.02 x
... 31.) Write electron configuration for nickel and selenium. 32.) Draw orbital notation for H.O.E.L. of nickel and selenium. 33.) Write noble gas configuration for nickel and selenium. 34.) Write out the four quantum numbers for the last electron added to nickel and selenium. (Honors only) 35.) Draw t ...
... 31.) Write electron configuration for nickel and selenium. 32.) Draw orbital notation for H.O.E.L. of nickel and selenium. 33.) Write noble gas configuration for nickel and selenium. 34.) Write out the four quantum numbers for the last electron added to nickel and selenium. (Honors only) 35.) Draw t ...
04-Atoms_ molecules_ ions_etc
... Atomic Mass • The weighted average mass of all the isotopes of an element • average of relative abundance x mass number for each isotope ...
... Atomic Mass • The weighted average mass of all the isotopes of an element • average of relative abundance x mass number for each isotope ...
Chapter 10 Power Point - Biloxi Public Schools
... the # of p+ always equals the # of ein an atom. ...
... the # of p+ always equals the # of ein an atom. ...
Atoms and the Periodic Table
... (1869, Russian) • Organized elements by increasing atomic mass • Predicted the existence of undiscovered elements. ...
... (1869, Russian) • Organized elements by increasing atomic mass • Predicted the existence of undiscovered elements. ...
ionization energies
... element, you can gain an idea of how “willing” an atom is to lose an electron, and relate this to its reactivity • In the next slide, you will see data from an experiment in which the 1st ionization energies of elements are plotted against atomic number. ...
... element, you can gain an idea of how “willing” an atom is to lose an electron, and relate this to its reactivity • In the next slide, you will see data from an experiment in which the 1st ionization energies of elements are plotted against atomic number. ...
FIREWORKS EMC summary notes
... The most recently discovered elements have been made by scientists. These elements are found after uranium at the bottom of the Periodic Table. ...
... The most recently discovered elements have been made by scientists. These elements are found after uranium at the bottom of the Periodic Table. ...
Atom Building blocks of matter Proton Sub
... Sub-atomic particle with positive (+) charge; located in nucleus of atom; determines identity of element ...
... Sub-atomic particle with positive (+) charge; located in nucleus of atom; determines identity of element ...
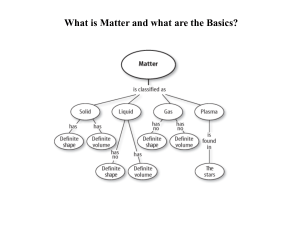
Matter: A) Homogeneous Matter • Uniform and in 1 phase • Even
... Rutherford: Gold foil experiment with alpha particles (positive) bombarding a piece of gold. Most of them went through the foil, but some were deflected. Shows that the atom is mostly empty space and has a small, positive, dense center (nucleus). It disproved Thomson. Also discovered alpha, beta, an ...
... Rutherford: Gold foil experiment with alpha particles (positive) bombarding a piece of gold. Most of them went through the foil, but some were deflected. Shows that the atom is mostly empty space and has a small, positive, dense center (nucleus). It disproved Thomson. Also discovered alpha, beta, an ...
ATOMS AND ELEMENTS
... A. Electrons travel around the nucleus in the electron cloud. B. Electrons follow paths called energy levels or energy shells. C. All elements have at least 1 energy level. D. The period number (or the rows) on the Periodic Table tells you the number of occupied energy shells that element has. E. El ...
... A. Electrons travel around the nucleus in the electron cloud. B. Electrons follow paths called energy levels or energy shells. C. All elements have at least 1 energy level. D. The period number (or the rows) on the Periodic Table tells you the number of occupied energy shells that element has. E. El ...
levels of organization and the atom
... unable to cut”. It has two areas: a nucleus and an electron cloud. It contains subatomic particles that are even smaller. ...
... unable to cut”. It has two areas: a nucleus and an electron cloud. It contains subatomic particles that are even smaller. ...
HONORS CHEMISTRY Quarter 2 Exam Topics Know the following
... o Democritus, Dalton, Thomson, Rutherford, Chadwick, Bohr, Schrodinger, Heisenberg Know experimental observations and their key contributions to the atomic theory. Be able to draw and label the model of the atom for each scientist mentioned above. Know Dalton’s postulates. Distinguish atoms ba ...
... o Democritus, Dalton, Thomson, Rutherford, Chadwick, Bohr, Schrodinger, Heisenberg Know experimental observations and their key contributions to the atomic theory. Be able to draw and label the model of the atom for each scientist mentioned above. Know Dalton’s postulates. Distinguish atoms ba ...
Atomic Models
... atoms of an element are different in mass and properties from those of any other element. 3. Atoms of on element cannot be ________________ into atoms of another element. 4. _________________ are the chemical combination of two or more elements in a specific ratio. For example: Was Dalton completely ...
... atoms of an element are different in mass and properties from those of any other element. 3. Atoms of on element cannot be ________________ into atoms of another element. 4. _________________ are the chemical combination of two or more elements in a specific ratio. For example: Was Dalton completely ...