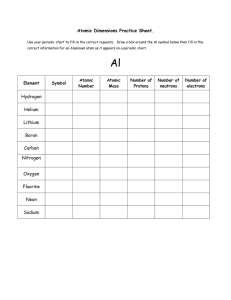
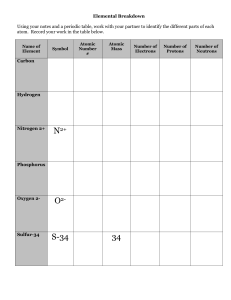
The atom - WordPress.com
... atomic theory based on many experiments. His was the first workable atomic theory. It stated that: All matter was made up of atoms. Atoms are indestructible. Each element has its own atom and that different elements had different types of atoms. Atoms of the same element are identical. ...
... atomic theory based on many experiments. His was the first workable atomic theory. It stated that: All matter was made up of atoms. Atoms are indestructible. Each element has its own atom and that different elements had different types of atoms. Atoms of the same element are identical. ...
chpt 11 and 12 notes with answers
... Location will help you predict how it will react with others in bonding. ...
... Location will help you predict how it will react with others in bonding. ...
Modern Atomic Theory and The Periodic Table
... Any level above ground state is called an _____________________________. The chemical properties of an element and its position on the periodic table depend on ________________________. Bohr’s ______________________________ for hydrogen does not work for any other element. ...
... Any level above ground state is called an _____________________________. The chemical properties of an element and its position on the periodic table depend on ________________________. Bohr’s ______________________________ for hydrogen does not work for any other element. ...
Trends in the periodic table - Brigham Young University
... M + H2O MOH (M = Li, Na, K, Rb, Cs) ...
... M + H2O MOH (M = Li, Na, K, Rb, Cs) ...
California Chemistry Standards Test
... 1. proteins, nucleic acids, and starch are made up of repetitive combinations 2. hydrocarbons and polymers 3. amino acids building blocks of matter Nuclear Processes-(2) 1. protons and neutrons in the nucleus (forces) 2. fusion and fission 3. isotopes of elements are radioactive 4. radioactive decay ...
... 1. proteins, nucleic acids, and starch are made up of repetitive combinations 2. hydrocarbons and polymers 3. amino acids building blocks of matter Nuclear Processes-(2) 1. protons and neutrons in the nucleus (forces) 2. fusion and fission 3. isotopes of elements are radioactive 4. radioactive decay ...
Part A: Multiple Choice. Circle the letter
... 7. In general, which of the following properties does NOT increase across a row from left to right? a) atomic number b) atomic radius c) nuclear charge d) ionization energy e) electron affinity 8. Which of the following properties decreases from top to bottom in a column? a) ionization energy b) ato ...
... 7. In general, which of the following properties does NOT increase across a row from left to right? a) atomic number b) atomic radius c) nuclear charge d) ionization energy e) electron affinity 8. Which of the following properties decreases from top to bottom in a column? a) ionization energy b) ato ...
Midterm Review File
... b. Identify the name of the group that contains the element fluorine _______________ c. Give the name of the element in the alkali group that has the greatest electron affinity _________. d. Identify the element in the oxygen group whose outermost electrons are in the fourth energy level____. e. Ide ...
... b. Identify the name of the group that contains the element fluorine _______________ c. Give the name of the element in the alkali group that has the greatest electron affinity _________. d. Identify the element in the oxygen group whose outermost electrons are in the fourth energy level____. e. Ide ...
example - Royal Society of Chemistry
... - Describe states of matter in terms of the arrangement & motion of simple ‘particles’ Use simple models to explain observations or processes: - change of state (s) (l) (g) requires heating - particles in the solid & liquid states are attracted to each other - energy transfer from the surroundin ...
... - Describe states of matter in terms of the arrangement & motion of simple ‘particles’ Use simple models to explain observations or processes: - change of state (s) (l) (g) requires heating - particles in the solid & liquid states are attracted to each other - energy transfer from the surroundin ...
Vocab: 1. Atamos 2. Indivisible 3. Indestructible 4. Atom 5. Electron 6
... 9. Chadwick- discovered the neutron -neutral particle -found in the nucleus 10. Ernest Rutherford -Gold Foil Experiment -Used alpha particles -Discovered the nucleus of the atom -Knew that it was positively charged -reasons- the results of his experiment a. most particles went through the foil b. so ...
... 9. Chadwick- discovered the neutron -neutral particle -found in the nucleus 10. Ernest Rutherford -Gold Foil Experiment -Used alpha particles -Discovered the nucleus of the atom -Knew that it was positively charged -reasons- the results of his experiment a. most particles went through the foil b. so ...
Atomic Structure Test Review Answer Key - Unit 1
... i. atomic number- indicates the number of protons in an atom. j. atomic mass- weighted average of all of the different isotopes of an element. k. atomic orbital- region of high probability of finding an electron l. aufbau principle- tendency of electrons to enter orbitals of lowest energy first ...
... i. atomic number- indicates the number of protons in an atom. j. atomic mass- weighted average of all of the different isotopes of an element. k. atomic orbital- region of high probability of finding an electron l. aufbau principle- tendency of electrons to enter orbitals of lowest energy first ...
Earth`s Chemistry
... there’s no charge. It’s said to be neutral Example --- Oxygen has an atomic number of 8 so it has 8 protons & 8 electrons mass number = protons + neutrons Protons & neutrons have an atomic mass of 1 ...
... there’s no charge. It’s said to be neutral Example --- Oxygen has an atomic number of 8 so it has 8 protons & 8 electrons mass number = protons + neutrons Protons & neutrons have an atomic mass of 1 ...
1 - M*W
... d) Have the same number of electrons 23) To draw a Lewis structure you do not need to know a) The number of valence electrons for each atom b) The types of atoms in the molecule c) The number of atoms in the molecule d) Bond energies 24) Neils Bohr’s contribution to modern atomic theory was the prop ...
... d) Have the same number of electrons 23) To draw a Lewis structure you do not need to know a) The number of valence electrons for each atom b) The types of atoms in the molecule c) The number of atoms in the molecule d) Bond energies 24) Neils Bohr’s contribution to modern atomic theory was the prop ...
Test Review: Unit 1 - Ms. Hill`s Pre
... normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain exactly the same elements in exactly the same proportions in exactly the same ...
... normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain exactly the same elements in exactly the same proportions in exactly the same ...
What does an elements atomic # tell us about the element?
... Smallest part of a compound that still has all the properties of that compound Compound can have properties entirely unlike the elements of which it is made ...
... Smallest part of a compound that still has all the properties of that compound Compound can have properties entirely unlike the elements of which it is made ...
Unit 2 Practice Exam exam_2p_08_matter
... 42. Why do atomic radii increase dramatically with each additional row of the periodic table? a. atomic nuclei become increasingly attractive as more protons are added. b. another energy level is utilized by the electrons. c. the energy required to remove an electron is reduced by shielding of inter ...
... 42. Why do atomic radii increase dramatically with each additional row of the periodic table? a. atomic nuclei become increasingly attractive as more protons are added. b. another energy level is utilized by the electrons. c. the energy required to remove an electron is reduced by shielding of inter ...
CHEMISTRY The Molecular Science
... • Atoms of different elements have different properties. • Compounds are formed when atoms of two or more elements combine. In a given compound, the relative number of atoms of each kind are definite and constant. • In an ordinary chemical reaction, no atom of any element disappears or is changed in ...
... • Atoms of different elements have different properties. • Compounds are formed when atoms of two or more elements combine. In a given compound, the relative number of atoms of each kind are definite and constant. • In an ordinary chemical reaction, no atom of any element disappears or is changed in ...
CH 5 Periodic Law
... - highly reactive metallic elements in group 1 - react with water to form hydrogen and alkaline solutions; burn in air - one outer electron, by losing this electron they become a cation, and become stable - soft metals; can be cut with a knife - shiny, but dull quickly due to oxygen and water in air ...
... - highly reactive metallic elements in group 1 - react with water to form hydrogen and alkaline solutions; burn in air - one outer electron, by losing this electron they become a cation, and become stable - soft metals; can be cut with a knife - shiny, but dull quickly due to oxygen and water in air ...
Name Date Class Period ______
... Name ______________________________________ Date __________________ Class Period _________ Atoms, Elements, and Compound Test Study Guide I. ...
... Name ______________________________________ Date __________________ Class Period _________ Atoms, Elements, and Compound Test Study Guide I. ...