Atomic Structure
... 4 parts of Dalton’s theory: 1. All elements are composed of tiny indivisible particles called atoms. 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms of different elements can physically mix together or can chemically c ...
... 4 parts of Dalton’s theory: 1. All elements are composed of tiny indivisible particles called atoms. 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms of different elements can physically mix together or can chemically c ...
Atoms and Electrons Review Sheet
... Quantum Mechanics – Energy comes in little quantifiable packets called quanta or photons (in the case of light) Alpha, Beta, Gamma Radiation Emission Spectrum vs. Absorbance Spectrum Electrons in the Atom Can you label a Periodic Table for use with electron configurations? Energy Levels / Shells (en ...
... Quantum Mechanics – Energy comes in little quantifiable packets called quanta or photons (in the case of light) Alpha, Beta, Gamma Radiation Emission Spectrum vs. Absorbance Spectrum Electrons in the Atom Can you label a Periodic Table for use with electron configurations? Energy Levels / Shells (en ...
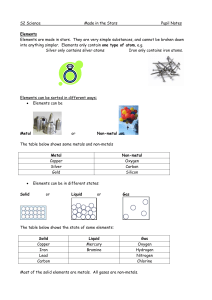
Made in the Stars Notes
... Alkali earth metals Group 7 Halogens Group 8 (or 0) Noble gases All of the elements in the same group have similar chemical properties, e.g. Group 1 metals are all very reactive, very soft and have to be stored under oil. Group 8 are all very unreactive gases. ...
... Alkali earth metals Group 7 Halogens Group 8 (or 0) Noble gases All of the elements in the same group have similar chemical properties, e.g. Group 1 metals are all very reactive, very soft and have to be stored under oil. Group 8 are all very unreactive gases. ...
Atomic Structure and Periodic Table Review Guide
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
Bohr – Rutherford Diagrams
... draw a circle around the nucleus. This represents the first energy level; draw one small dot on the first energy level line for each electron calculated in step 1 (up to a maximum of 2 dots). Each dot represents 1 electron; if the atom contains more than 2 electrons, draw a second circle around the ...
... draw a circle around the nucleus. This represents the first energy level; draw one small dot on the first energy level line for each electron calculated in step 1 (up to a maximum of 2 dots). Each dot represents 1 electron; if the atom contains more than 2 electrons, draw a second circle around the ...
Lesson x- Review W14 answers
... pudding -discovered charge in the atom Rutherford -atoms are mostly empty space (where electrons are) with a dense positive centre (nucleus) -used the gold foil experiment to discover the nucleus; most particles went through, few bounced back Bohr -atoms have a dense nucleus surround by shells (ener ...
... pudding -discovered charge in the atom Rutherford -atoms are mostly empty space (where electrons are) with a dense positive centre (nucleus) -used the gold foil experiment to discover the nucleus; most particles went through, few bounced back Bohr -atoms have a dense nucleus surround by shells (ener ...
Review Sheet Filled Out
... List the number of facts you know about electrons. Electrons closest to the nucleus have the least amount of energy Electrons farthest away from the nucleus have the most energy – valence e Have a negative charge Have insignificant mass and volume Reside in the 99.996% of the atom outside t ...
... List the number of facts you know about electrons. Electrons closest to the nucleus have the least amount of energy Electrons farthest away from the nucleus have the most energy – valence e Have a negative charge Have insignificant mass and volume Reside in the 99.996% of the atom outside t ...
Atomic Structure
... •Unstable isotopes called radioisotopes undergo changes and release energy to become more stable. These isotopes have many uses which we will discuss in the Nuclear Chemistry section of our class. Now, let’s compare the three isotopes of Lithium! How many protons does Lithium-6 have? ________ How ma ...
... •Unstable isotopes called radioisotopes undergo changes and release energy to become more stable. These isotopes have many uses which we will discuss in the Nuclear Chemistry section of our class. Now, let’s compare the three isotopes of Lithium! How many protons does Lithium-6 have? ________ How ma ...
Test #5 Review
... Which force holds the nucleus together? the strong force Which force holds the electrons around the nucleus? the electromagnetic force Define mass number. number of protons + number of neutrons ...
... Which force holds the nucleus together? the strong force Which force holds the electrons around the nucleus? the electromagnetic force Define mass number. number of protons + number of neutrons ...
ATOMIC THEORY
... Most of the mass of the atom and all of its positive charge is contained in a tiny core region called the nucleus The nucleus contains protons and neutrons (Chadwick, 1932) that have approximately the same mass The number of protons is the atomic number (Z) The total number of protons and ne ...
... Most of the mass of the atom and all of its positive charge is contained in a tiny core region called the nucleus The nucleus contains protons and neutrons (Chadwick, 1932) that have approximately the same mass The number of protons is the atomic number (Z) The total number of protons and ne ...
5 - BrainMass
... smaller than that for a 3p electron. In light of this fact, which orbital is higher in energy? b. Would you expect it to require more or less energy to remove a 3s electron from the chlorine atom, as compared with a 2p electron? Explain. ...
... smaller than that for a 3p electron. In light of this fact, which orbital is higher in energy? b. Would you expect it to require more or less energy to remove a 3s electron from the chlorine atom, as compared with a 2p electron? Explain. ...
The Nature of Matter
... • Balances out protons positive charge • In constant motion • Valence electrons are in outermost shell • Valence electrons determine the chemical nature of an atom • Smallest subatomic particle ...
... • Balances out protons positive charge • In constant motion • Valence electrons are in outermost shell • Valence electrons determine the chemical nature of an atom • Smallest subatomic particle ...
Electron Arrangement
... The test for oxygen is it relights a glowing flame. The main components of air are oxygen and nitrogen in proportion of 1:4. An exothermic reaction is one in which energy has been released (given out). This feels hot to the touch. Finite energy resources will run out. This means there will be a fuel ...
... The test for oxygen is it relights a glowing flame. The main components of air are oxygen and nitrogen in proportion of 1:4. An exothermic reaction is one in which energy has been released (given out). This feels hot to the touch. Finite energy resources will run out. This means there will be a fuel ...
Nuclear Chemistry Worksheet
... orbitals oriented in different directions) I.e. in the p orbital, there are three orbitals. For ...
... orbitals oriented in different directions) I.e. in the p orbital, there are three orbitals. For ...
Chemistry Test Review - Greenslime Home Page
... 8. List and describe the 3 subatomic particles. Include location, mass and charge. a. Proton – located in the nucleus – mass of 1 amu – charge is positive (+) b. Neutron – located in the nucleus – mass of 1 amu – charge is neutral (0) c. Electron – located in electron cloud (shells, orbitals) – mass ...
... 8. List and describe the 3 subatomic particles. Include location, mass and charge. a. Proton – located in the nucleus – mass of 1 amu – charge is positive (+) b. Neutron – located in the nucleus – mass of 1 amu – charge is neutral (0) c. Electron – located in electron cloud (shells, orbitals) – mass ...
Chemistry Review- Answer all questions on loose
... reactivity increases as you move down the group. Calcium is less reactive as it is in period 4 and Barium is in period 6. b) Boron or Argon - Boron is more reactive than argon since argon is a noble gas. Noble gases are the least reactive elements since they have complete outer shells. Boron is also ...
... reactivity increases as you move down the group. Calcium is less reactive as it is in period 4 and Barium is in period 6. b) Boron or Argon - Boron is more reactive than argon since argon is a noble gas. Noble gases are the least reactive elements since they have complete outer shells. Boron is also ...
Chapter 4 Atomic Structure I. History of the Atom A. Democritus (400
... 1. Ground state: All the electrons in an atom have the lowest possible energy a. Stable 2. Excited state: An electron moves to an orbital of higher energy a. Less stable Chapter 5 Periodic Table I. Organizing the elements A. Mendeleev’s Periodic Table 1. Arranged in rows by increasing atomic mass 2. ...
... 1. Ground state: All the electrons in an atom have the lowest possible energy a. Stable 2. Excited state: An electron moves to an orbital of higher energy a. Less stable Chapter 5 Periodic Table I. Organizing the elements A. Mendeleev’s Periodic Table 1. Arranged in rows by increasing atomic mass 2. ...
CHEMISTRY
... The nature of most atoms is that they are LONELY and sometimes AGGRESSIVE! Most atoms team up with or overtake other atoms in an attempt to get the “right” number of electrons. This is how molecules are formed. Only the NOBLE GASSES can exist on their own. ATOMS will switch partners when provoked. T ...
... The nature of most atoms is that they are LONELY and sometimes AGGRESSIVE! Most atoms team up with or overtake other atoms in an attempt to get the “right” number of electrons. This is how molecules are formed. Only the NOBLE GASSES can exist on their own. ATOMS will switch partners when provoked. T ...
Bohr Model & Lewis Dot Diagrams
... In the center are circles. Each circle represents a single neutron or proton. Protons should have a plus or P written on them. Neutrons should be blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
... In the center are circles. Each circle represents a single neutron or proton. Protons should have a plus or P written on them. Neutrons should be blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
Physical Science 1st Semester final Review
... 134. Suppose you want to separate the leaves, acorns, and twigs from a pile of soil. Filtration and distillation are two processes of separating mixtures. Explain which process you would use and why. ...
... 134. Suppose you want to separate the leaves, acorns, and twigs from a pile of soil. Filtration and distillation are two processes of separating mixtures. Explain which process you would use and why. ...
Metric Unit – Chapter 1
... 4. Atoms of different elements combine in simple wholenumbered ratios to form 5. In chemical reactions, atoms ___________________________. are _____________________ 5. In chemical reactions, atoms are _______________________. ___________________________ ___________________________. ...
... 4. Atoms of different elements combine in simple wholenumbered ratios to form 5. In chemical reactions, atoms ___________________________. are _____________________ 5. In chemical reactions, atoms are _______________________. ___________________________ ___________________________. ...
notes 4.1 & 4.2
... • Outermost ones are called valence electrons. They are responsible for how elements react with each other and the physical and chemical properties. ...
... • Outermost ones are called valence electrons. They are responsible for how elements react with each other and the physical and chemical properties. ...
CH 3 Atomic Structure Review-New
... 71. The law of multiple proportions states that whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other ...
... 71. The law of multiple proportions states that whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other ...
Unit 1: Introduction to Chemistry - Teach-n-Learn-Chem
... 71. The law of multiple proportions states that whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other ...
... 71. The law of multiple proportions states that whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other ...
The History of Atomic Theory
... Described the atom as a small, incompressible sphere with an atmosphere of heat. ...
... Described the atom as a small, incompressible sphere with an atmosphere of heat. ...