TEST-Periodic Table
... 29. Classifying Classify the elements in Figure 5-3 as metals, metalloids, or nonmetals. Explain your answer. a. These elements are all metals. They are found on the left side of the periodic table. b. These elements are all metals. They are found on the right side of the periodic table. c. These el ...
... 29. Classifying Classify the elements in Figure 5-3 as metals, metalloids, or nonmetals. Explain your answer. a. These elements are all metals. They are found on the left side of the periodic table. b. These elements are all metals. They are found on the right side of the periodic table. c. These el ...
The Nuts and Bolts of Periodic Tables
... students the fastener that was removed from their packet. Ask them where they would put it. What's going on: Hardware stores need to organize their fasteners so that customers can easily find and purchase them. In the case of fasteners, they can be organize by their physical properties and by how th ...
... students the fastener that was removed from their packet. Ask them where they would put it. What's going on: Hardware stores need to organize their fasteners so that customers can easily find and purchase them. In the case of fasteners, they can be organize by their physical properties and by how th ...
Chapter 6
... b. Hg d. Te How does atomic radius change from top to bottom in a group in the periodic table? a. It tends to decrease. c. It first increases, then decreases. b. It tends to increase. d. It first decreases, then increases. How does atomic radius change from left to right across a period in the perio ...
... b. Hg d. Te How does atomic radius change from top to bottom in a group in the periodic table? a. It tends to decrease. c. It first increases, then decreases. b. It tends to increase. d. It first decreases, then increases. How does atomic radius change from left to right across a period in the perio ...
The perfect K-12 presentation ever (replace this with your title)
... After hydrogen fusion, larger stars can continue with the fusion of ...
... After hydrogen fusion, larger stars can continue with the fusion of ...
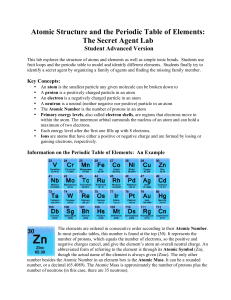
Atomic Structure and the Periodic Table of Elements: The Secret
... Today, the elements of the Periodic Table are arranged by atomic number, which also indicates the number of protons in an atom (see Periodic Table Tutorial above). Neutrons are uncharged particles in the atom’s nucleus that only affect the overall weight of the atom, not the charge. The Periodic Tab ...
... Today, the elements of the Periodic Table are arranged by atomic number, which also indicates the number of protons in an atom (see Periodic Table Tutorial above). Neutrons are uncharged particles in the atom’s nucleus that only affect the overall weight of the atom, not the charge. The Periodic Tab ...
Periodic Trends
... Many properties of the elements tend to change in a predictable way, known as trends. Trends among elements in the periodic table include their sizes and their abilities to lose or attract electrons. Atomic Size Definition: Atomic size is related the atomic radius of an element, which is defined as ...
... Many properties of the elements tend to change in a predictable way, known as trends. Trends among elements in the periodic table include their sizes and their abilities to lose or attract electrons. Atomic Size Definition: Atomic size is related the atomic radius of an element, which is defined as ...
Chapter 6 Study Guide Key
... Electronegativity is the ability of an atom to pull electrons towards itself in a covalent bond. Think of it like a tug of war. 19.What is the trend in electronegativity down a group on the periodic table? What causes this trend? Electronegativity decreases as you go down a group on the periodic tab ...
... Electronegativity is the ability of an atom to pull electrons towards itself in a covalent bond. Think of it like a tug of war. 19.What is the trend in electronegativity down a group on the periodic table? What causes this trend? Electronegativity decreases as you go down a group on the periodic tab ...
The Periodic Law Notes (Chapter 5) – Part 2
... because the atoms get smaller. Another way to think of it: the number of valence electrons increases (the amount of energy needed to remove one electron is less then what is needed to remove 7 or 8 electrons). 3. Group trend – ionization energy increases as you move up a group (or decreases as you m ...
... because the atoms get smaller. Another way to think of it: the number of valence electrons increases (the amount of energy needed to remove one electron is less then what is needed to remove 7 or 8 electrons). 3. Group trend – ionization energy increases as you move up a group (or decreases as you m ...
Chapter 5 study guide - Peoria Public Schools
... 3. Describe how Mendeleev's periodic table is organized. 4. Explain was wrong with Mendeleev's periodic law? 5. State the modern periodic law 6. Describe how the modern periodic table is organized. 7. Define: period, series, group, family. 8. Explain why elements in the same family have similar prop ...
... 3. Describe how Mendeleev's periodic table is organized. 4. Explain was wrong with Mendeleev's periodic law? 5. State the modern periodic law 6. Describe how the modern periodic table is organized. 7. Define: period, series, group, family. 8. Explain why elements in the same family have similar prop ...
Alkaline Earth Metals
... How are sublevels and PELs related? How are orbitals and orbits related? What’s the formula for the maximum number of electrons allowed in a certain PEL? List the elements that exist as diatomics. What are the trends in atomic radius as you go down and across the periodic table? Why? What are the tr ...
... How are sublevels and PELs related? How are orbitals and orbits related? What’s the formula for the maximum number of electrons allowed in a certain PEL? List the elements that exist as diatomics. What are the trends in atomic radius as you go down and across the periodic table? Why? What are the tr ...
CHEM 1405 CHAPTER 5
... shells in the valence shell. 3. Transition Metals ( d - block elements) They are the elements of the groups IB and 3B through 8B. All of them are metals with incompletely filled (n-1) d sub shells. Elements of 2B have completely filled (n-1) d sub shells. But are studied along with transition metals ...
... shells in the valence shell. 3. Transition Metals ( d - block elements) They are the elements of the groups IB and 3B through 8B. All of them are metals with incompletely filled (n-1) d sub shells. Elements of 2B have completely filled (n-1) d sub shells. But are studied along with transition metals ...
The Periodic Table and Periodic Law
... 1869: Meyer and Mendeleev each demonstrated a connection between atomic mass and elemental properties. – Mendeleev published his scheme first and thus received more credit. – Mendeleev arranged elements in columns of increasing atomic mass since the elements had similar properties… this developed in ...
... 1869: Meyer and Mendeleev each demonstrated a connection between atomic mass and elemental properties. – Mendeleev published his scheme first and thus received more credit. – Mendeleev arranged elements in columns of increasing atomic mass since the elements had similar properties… this developed in ...
Chapter 6 Practice Test
... b. Hg d. Te How does atomic radius change from top to bottom in a group in the periodic table? a. It tends to decrease. c. It first increases, then decreases. b. It tends to increase. d. It first decreases, then increases. How does atomic radius change from left to right across a period in the perio ...
... b. Hg d. Te How does atomic radius change from top to bottom in a group in the periodic table? a. It tends to decrease. c. It first increases, then decreases. b. It tends to increase. d. It first decreases, then increases. How does atomic radius change from left to right across a period in the perio ...
Periodic Table
... John Newlands John Newlands (1837 – 1898) proposed the law of octaves (~1865) It states; if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements. Meaning every eighth element shows s ...
... John Newlands John Newlands (1837 – 1898) proposed the law of octaves (~1865) It states; if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements. Meaning every eighth element shows s ...
Introductory Chemistry: Concepts & Connections 4th Edition
... • In general, the ionization energy increases as you go from the bottom to the top in a group. • In general, the ionization energy increases as you go from left to right across a period of elements. • The closer the electron to the nucleus, the more energy is required to remove the electron. Chapter ...
... • In general, the ionization energy increases as you go from the bottom to the top in a group. • In general, the ionization energy increases as you go from left to right across a period of elements. • The closer the electron to the nucleus, the more energy is required to remove the electron. Chapter ...
Periodic Table
... • In general, the ionization energy increases as you go from the bottom to the top in a group. • In general, the ionization energy increases as you go from left to right across a period of elements. • The closer the electron to the nucleus, the more energy is required to remove the electron. Chapter ...
... • In general, the ionization energy increases as you go from the bottom to the top in a group. • In general, the ionization energy increases as you go from left to right across a period of elements. • The closer the electron to the nucleus, the more energy is required to remove the electron. Chapter ...
D. - Taylor County Schools
... atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the e ...
... atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the e ...
Chapter 5- The Periodic Law
... B. Higher melting points C. Too reactive to be found in nature as free elements. D. Hydrogen and Helium 1. Hydrogen does not share the same properties as the elements in Group 1 2. Helium possesses special chemical stability because of its filled outer shell E. The d-Block Elements: Groups 3-12 ...
... B. Higher melting points C. Too reactive to be found in nature as free elements. D. Hydrogen and Helium 1. Hydrogen does not share the same properties as the elements in Group 1 2. Helium possesses special chemical stability because of its filled outer shell E. The d-Block Elements: Groups 3-12 ...
version
... Due to the fact that there are then more protons than electrons, and the stronger positive charge will then act on the remaining electrons to hold them to the atom (Remember that the charge on the nucleus increases while the charge on each electron remains the same, causing more pull by the nucleu ...
... Due to the fact that there are then more protons than electrons, and the stronger positive charge will then act on the remaining electrons to hold them to the atom (Remember that the charge on the nucleus increases while the charge on each electron remains the same, causing more pull by the nucleu ...
The Periodic Table and Periodic Law
... What does a group tell us about valence electrons and ion formation? ...
... What does a group tell us about valence electrons and ion formation? ...
Homework Answers - Chemistry from AZ
... horizontal rows called periods are numbered 1 to 7; elements in the same period have the same number of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have ...
... horizontal rows called periods are numbered 1 to 7; elements in the same period have the same number of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have ...
Chapter 6
... • In 1829, the German chemist J.W. Döbereiner observed that several elements could be classified into groups of three, or triads. • All three elements in a triad showed very similar chemical properties and an orderly trend in physical properties. ...
... • In 1829, the German chemist J.W. Döbereiner observed that several elements could be classified into groups of three, or triads. • All three elements in a triad showed very similar chemical properties and an orderly trend in physical properties. ...
The Periodic Law - Mona Shores Blogs
... 5-1 History of the Periodic Table Objective: Covers the work of Mendeleev and other chemists in developing the periodic table and explains how the periodic law is used to predict elements’ physical and chemical properties. Mendeleev and Chemical Periodicity Dmitri Mendeleev noticed that when eleme ...
... 5-1 History of the Periodic Table Objective: Covers the work of Mendeleev and other chemists in developing the periodic table and explains how the periodic law is used to predict elements’ physical and chemical properties. Mendeleev and Chemical Periodicity Dmitri Mendeleev noticed that when eleme ...
Group 3 element

Group 3 is a group of elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group. Scandium (Sc) and yttrium (Y) are always included, but the other two spaces are usually occupied by lanthanum (La) and actinium (Ac), or by lutetium (Lu) and lawrencium (Lr); less frequently, it is considered the group should be expanded to 32 elements (with all the lanthanides and actinides included) or contracted to contain only scandium and yttrium. The group itself has not acquired a trivial name; however, scandium, yttrium and the lanthanides are sometimes called rare earth metals.Three group 3 elements occur naturally, scandium, yttrium, and either lanthanum or lutetium. Lanthanum continues the trend started by two lighter members in general chemical behavior, while lutetium behaves more similarly to yttrium. This is in accordance with the trend for period 6 transition metals to behave more similarly to their upper periodic table neighbors. This trend is seen from hafnium, which is almost identical chemically to zirconium, to mercury, which is quite distant chemically from cadmium, but still shares with it almost equal atomic size and other similar properties. They all are silvery-white metals under standard conditions. The fourth element, either actinium or lawrencium, has only radioactive isotopes. Actinium, which occurs only in trace amounts, continues the trend in chemical behavior for metals that form tripositive ions with a noble gas configuration; synthetic lawrencium is calculated and partially shown to be more similar to lutetium and yttrium. So far, no experiments have been conducted to synthesize any element that could be the next group 3 element. Unbiunium (Ubu), which could be considered a group 3 element if preceded by lanthanum and actinium, might be synthesized in the near future, it being only three spaces away from the current heaviest element known, ununoctium.