Homework Packet - Chemistry from AZ
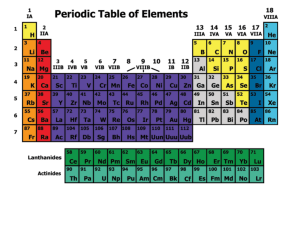
... horizontal rows called periods are numbered 1 to 7; elements in the same period have the same number of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have ...
... horizontal rows called periods are numbered 1 to 7; elements in the same period have the same number of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have ...
Periodic Law
... Group 1 – (alkali metals) are all highly reactive and are rarely found in elemental form in nature Group 2 – (alkaline earth metals) are silvery colored, soft metals ...
... Group 1 – (alkali metals) are all highly reactive and are rarely found in elemental form in nature Group 2 – (alkaline earth metals) are silvery colored, soft metals ...
Chapter 1
... Group 1 – (alkali metals) are all highly reactive and are rarely found in elemental form in nature Group 2 – (alkaline earth metals) are silvery colored, soft metals ...
... Group 1 – (alkali metals) are all highly reactive and are rarely found in elemental form in nature Group 2 – (alkaline earth metals) are silvery colored, soft metals ...
Periodic Table
... • Each period begins with a new outer electron orbital • Each period ends with a completely filled outer orbital that has the maximum number of electrons for that orbital. ...
... • Each period begins with a new outer electron orbital • Each period ends with a completely filled outer orbital that has the maximum number of electrons for that orbital. ...
Unit 4 (2016 – 2017)
... 1869 – ________________________________ (Russian chemist) organized elements by atomic _________ also but made it into table form to help his students. Elements with similar properties were put in the same___________. He was considered the ____________ of the Modern Periodic Table. He left blanks wh ...
... 1869 – ________________________________ (Russian chemist) organized elements by atomic _________ also but made it into table form to help his students. Elements with similar properties were put in the same___________. He was considered the ____________ of the Modern Periodic Table. He left blanks wh ...
Chapter 5 – The Periodic Law
... periodic table in groups • 2. Elements are arranged horizontally on the periodic table in periods • 3. There are 8 • groups and 7 • periods of • elements on • the periodic • table ...
... periodic table in groups • 2. Elements are arranged horizontally on the periodic table in periods • 3. There are 8 • groups and 7 • periods of • elements on • the periodic • table ...
Monday - Houston ISD
... Standards does it support? - How does it support the Readiness Standards? I will know my students have mastered this standard when they can…. ...
... Standards does it support? - How does it support the Readiness Standards? I will know my students have mastered this standard when they can…. ...
STUDY GUIDE – CHAPTER 1 ATOMS AND ELEMENTS 1
... An element is examined in the laboratory. It is found to be a gas at room temperature. Also it reacts, vigorously with some metals. To which family or group does the element belong? ___________________________________________ ...
... An element is examined in the laboratory. It is found to be a gas at room temperature. Also it reacts, vigorously with some metals. To which family or group does the element belong? ___________________________________________ ...
First Term Science Al-Karma Language School Prep 2 Question (1
... weight, while Moseley arranged them ascending according to atomic number. 10)-Calcium (Ca) and Magnesium (Mg) elements are examples of alkaline earth metals. 11)-The valency energy level of halogen contains seven electrons, while that of alkaline earth metal has two electrons. 12)-Sodium and potassi ...
... weight, while Moseley arranged them ascending according to atomic number. 10)-Calcium (Ca) and Magnesium (Mg) elements are examples of alkaline earth metals. 11)-The valency energy level of halogen contains seven electrons, while that of alkaline earth metal has two electrons. 12)-Sodium and potassi ...
Atomic structure and Periodic table revision guide File
... called the noble gases. They are unreactive and do not easily form molecules because their atoms have stable arrangements of electrons. The noble gases have eight electrons in their outer energy level, except for helium, which has only two electrons. The boiling points of the noble gases increase wi ...
... called the noble gases. They are unreactive and do not easily form molecules because their atoms have stable arrangements of electrons. The noble gases have eight electrons in their outer energy level, except for helium, which has only two electrons. The boiling points of the noble gases increase wi ...
The Periodic Table of the Elements
... electrons to form chemical bonds • As you go across a period from left to right, the nuclear charge increases while the number of energy levels stays the same, so there is a stronger and stronger attraction for the electrons. It becomes more and more difficult to lose electrons, so the reactivity of ...
... electrons to form chemical bonds • As you go across a period from left to right, the nuclear charge increases while the number of energy levels stays the same, so there is a stronger and stronger attraction for the electrons. It becomes more and more difficult to lose electrons, so the reactivity of ...
unit iv – the periodic table
... The periodic table is the arrangement of the elements in order of their atomic numbers so that elements with similar properties fall in the same column or group. Noble Gases were added to the periodic table later - early 1900's Lanthanides - f-Block was added in the early 1900's after the identity ...
... The periodic table is the arrangement of the elements in order of their atomic numbers so that elements with similar properties fall in the same column or group. Noble Gases were added to the periodic table later - early 1900's Lanthanides - f-Block was added in the early 1900's after the identity ...
Chapter 3 Physical Science - St. Pius X Classical Academy
... Introduction to Atoms Rutherford’s Gold Foil Experiment Rutherford was surprised that a few particles were deflected strongly. Which are the paths of the particles that were not predicted by Thomson’s atomic model? ...
... Introduction to Atoms Rutherford’s Gold Foil Experiment Rutherford was surprised that a few particles were deflected strongly. Which are the paths of the particles that were not predicted by Thomson’s atomic model? ...
Week 21 Lessons - Highline Public Schools
... - # of protons = Atomic Number - # of electrons = # of protons (this is a neutral atom.) - Mass number = # of protons + # of neutrons - # of neutrons = mass number - # of protons (Mass number must be a whole number!!!! You can’t have half a neutron. Use atomic mass and round up.) Exit Ticket: The nu ...
... - # of protons = Atomic Number - # of electrons = # of protons (this is a neutral atom.) - Mass number = # of protons + # of neutrons - # of neutrons = mass number - # of protons (Mass number must be a whole number!!!! You can’t have half a neutron. Use atomic mass and round up.) Exit Ticket: The nu ...
Standards Practice
... A. They have only one electron in their outer electron shells, so they frequently form singly charged positive ions. B. They have only two electrons in their outer shells, so they frequently bond with doubly ...
... A. They have only one electron in their outer electron shells, so they frequently form singly charged positive ions. B. They have only two electrons in their outer shells, so they frequently bond with doubly ...
Appendices: Cluster 2 Atoms and Elements
... that contained few electrons, but it did not explain the more complex atoms. The discovery that particles sometimes exhibit wave properties, called the wave-particle duality, has led to the currently accepted theory of atomic structure called quantum mechanics. The Quantum model proposed the followi ...
... that contained few electrons, but it did not explain the more complex atoms. The discovery that particles sometimes exhibit wave properties, called the wave-particle duality, has led to the currently accepted theory of atomic structure called quantum mechanics. The Quantum model proposed the followi ...
Chapter 3: Atoms & the Periodic Table
... • Because it is based on the structure of atoms, especially the valence electrons. –Elements in a family all have the same number of valence electrons • This is a reason why the elements in a particular group have similar properties ...
... • Because it is based on the structure of atoms, especially the valence electrons. –Elements in a family all have the same number of valence electrons • This is a reason why the elements in a particular group have similar properties ...
Chapter 7 The Development of the Periodic Table
... His research was halted when the British government sent him to serve as a foot soldier in WWI. He was killed in the fighting in Gallipoli by a sniper’s bullet, at the age of 28. Because of this loss, the ...
... His research was halted when the British government sent him to serve as a foot soldier in WWI. He was killed in the fighting in Gallipoli by a sniper’s bullet, at the age of 28. Because of this loss, the ...
The Periodic Table
... • In 1829, the German chemist J. W. Döbereiner observed that several elements could be classified into groups of three, or triads. • All three elements in a triad showed very similar chemical properties and an orderly trend in physical properties. ...
... • In 1829, the German chemist J. W. Döbereiner observed that several elements could be classified into groups of three, or triads. • All three elements in a triad showed very similar chemical properties and an orderly trend in physical properties. ...
Chapter 7 Periodic Properties of the Elements
... (a). less efficient than that by core electrons (b). more efficient than that by core electrons (c). essentially identical to that by core electrons (d). both more efficient than that by core electrons and responsible for a general increase in atomic radius going across a period. Explanation: Screen ...
... (a). less efficient than that by core electrons (b). more efficient than that by core electrons (c). essentially identical to that by core electrons (d). both more efficient than that by core electrons and responsible for a general increase in atomic radius going across a period. Explanation: Screen ...
Group 3 element

Group 3 is a group of elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group. Scandium (Sc) and yttrium (Y) are always included, but the other two spaces are usually occupied by lanthanum (La) and actinium (Ac), or by lutetium (Lu) and lawrencium (Lr); less frequently, it is considered the group should be expanded to 32 elements (with all the lanthanides and actinides included) or contracted to contain only scandium and yttrium. The group itself has not acquired a trivial name; however, scandium, yttrium and the lanthanides are sometimes called rare earth metals.Three group 3 elements occur naturally, scandium, yttrium, and either lanthanum or lutetium. Lanthanum continues the trend started by two lighter members in general chemical behavior, while lutetium behaves more similarly to yttrium. This is in accordance with the trend for period 6 transition metals to behave more similarly to their upper periodic table neighbors. This trend is seen from hafnium, which is almost identical chemically to zirconium, to mercury, which is quite distant chemically from cadmium, but still shares with it almost equal atomic size and other similar properties. They all are silvery-white metals under standard conditions. The fourth element, either actinium or lawrencium, has only radioactive isotopes. Actinium, which occurs only in trace amounts, continues the trend in chemical behavior for metals that form tripositive ions with a noble gas configuration; synthetic lawrencium is calculated and partially shown to be more similar to lutetium and yttrium. So far, no experiments have been conducted to synthesize any element that could be the next group 3 element. Unbiunium (Ubu), which could be considered a group 3 element if preceded by lanthanum and actinium, might be synthesized in the near future, it being only three spaces away from the current heaviest element known, ununoctium.