CHAPTER-3 CLASSIFICATION OF ELEMENTS
... property of an element than its atomic mass. Therefore, the position of an element in the periodic table depends on its atomic number than its atomic mass. Modern Periodic Law: The physical and chemical properties of elements are the periodic functions of their atomic numbers. Types of Elements: s-, ...
... property of an element than its atomic mass. Therefore, the position of an element in the periodic table depends on its atomic number than its atomic mass. Modern Periodic Law: The physical and chemical properties of elements are the periodic functions of their atomic numbers. Types of Elements: s-, ...
Chapter TWO
... Chemical (molecular) formula : It is a simple symbolic formula shows the type and the number of atoms or ions in the molecule of a compound . Emperical formula : It is the formula which represents the simplest composition percent of elements in a compound . C6H12O6 CH2O ...
... Chemical (molecular) formula : It is a simple symbolic formula shows the type and the number of atoms or ions in the molecule of a compound . Emperical formula : It is the formula which represents the simplest composition percent of elements in a compound . C6H12O6 CH2O ...
periods - Madeira City Schools
... good conductors of heat and electricity. ¥ Alkali metals are all the elements in group 1 except hydrogen, and are very reactive. ¥ Alkaline earth metals are in group 2, and are also highly reactive. ...
... good conductors of heat and electricity. ¥ Alkali metals are all the elements in group 1 except hydrogen, and are very reactive. ¥ Alkaline earth metals are in group 2, and are also highly reactive. ...
Chapter 7 - HCC Learning Web
... • Elements in the same column contain the same number of outer-shell electrons or valence electrons. • How do we organize the different elements in a meaningful way that will allow us to make predictions about undiscovered elements? • Arrange elements to reflect the trends in chemical and physical p ...
... • Elements in the same column contain the same number of outer-shell electrons or valence electrons. • How do we organize the different elements in a meaningful way that will allow us to make predictions about undiscovered elements? • Arrange elements to reflect the trends in chemical and physical p ...
February 10 Clicker Questions
... 1. [Ne]3p7 2. [Ne]3s23p5 3. [Ne]3s23p6 4. [Ne]3s23d5 5. [Ne]3s23p33d2 Note: 1 is impossible (p-orbitals can hold a maximum of 6 electrons), 4 and 5 are not ground state configurations, while 3 has 18 electrons. ...
... 1. [Ne]3p7 2. [Ne]3s23p5 3. [Ne]3s23p6 4. [Ne]3s23d5 5. [Ne]3s23p33d2 Note: 1 is impossible (p-orbitals can hold a maximum of 6 electrons), 4 and 5 are not ground state configurations, while 3 has 18 electrons. ...
c1l2ch06
... - Mendeleev's arrangement was not completely uniform, some elements were apparently 'out of place'. Note the masses of Te and I in Mendeleev's table. - Moseley discovered that the nuclear charge increased for each increase of atomic mass. ...
... - Mendeleev's arrangement was not completely uniform, some elements were apparently 'out of place'. Note the masses of Te and I in Mendeleev's table. - Moseley discovered that the nuclear charge increased for each increase of atomic mass. ...
6.1 Development of the Modern Periodic Table Objectives: 1
... electrons for that element Valence Electrons and Group Number The Roman numerals on the periodic table for the main-group elements show the number of valence electrons available for bonding The number of valence electrons for the transition elements is technically 2, but in reality the electro ...
... electrons for that element Valence Electrons and Group Number The Roman numerals on the periodic table for the main-group elements show the number of valence electrons available for bonding The number of valence electrons for the transition elements is technically 2, but in reality the electro ...
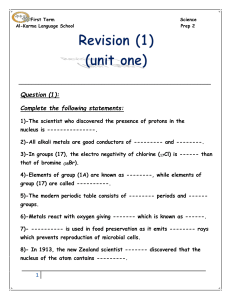
First Term Science Al-Karma Language School Prep 2 Question (1
... 11)-The valency energy level of halogen contains -------- electrons, while that of alkaline earth metal has --------- electrons. 12)-Sodium and potassium are kept under the surface of --------- to prevent them from the reaction with ----------. 13)-Each period in the modern periodic table starts wit ...
... 11)-The valency energy level of halogen contains -------- electrons, while that of alkaline earth metal has --------- electrons. 12)-Sodium and potassium are kept under the surface of --------- to prevent them from the reaction with ----------. 13)-Each period in the modern periodic table starts wit ...
Ch 4-1 Notes
... * Stored under kerosene to protect them from reacting with the moisture in the air. * Moving down the column the melting points decrease. Group 2 = alkaline earth metals * each element has two electrons in an s sublevel * tend to lose two electrons to form a +2 ion and achieve an octet. * harder, d ...
... * Stored under kerosene to protect them from reacting with the moisture in the air. * Moving down the column the melting points decrease. Group 2 = alkaline earth metals * each element has two electrons in an s sublevel * tend to lose two electrons to form a +2 ion and achieve an octet. * harder, d ...
Periodic Table Notes Ch. 6 ppt
... For a period (row), the number of shielding electrons remain the same, but the number of protons in the nucleus increases. Example: All elements in the second period have the same underlying [He] noble gas configuration. However, the number of protons increase from left to ...
... For a period (row), the number of shielding electrons remain the same, but the number of protons in the nucleus increases. Example: All elements in the second period have the same underlying [He] noble gas configuration. However, the number of protons increase from left to ...
Day 3
... periodic table. The guide will include a picture of an example block with information on each component (atomic number, atomic symbol, atomic mass). Explain -- Time Estimate: 20 minutes Each student will be assigned an element from the periodic table to make a poster about. The poster will include: ...
... periodic table. The guide will include a picture of an example block with information on each component (atomic number, atomic symbol, atomic mass). Explain -- Time Estimate: 20 minutes Each student will be assigned an element from the periodic table to make a poster about. The poster will include: ...
Question (1): Explain `Dobereiner`s Triads and its drawback. Answer
... 2) It was also observed that dissimilar elements were being grouped into triads. Question (2): State Newland's 'Law of Octaves'. Answer: When elements are arranged in ascending order of their atomic weights, every eighth element had similar physical and chemical properties and resembled the first el ...
... 2) It was also observed that dissimilar elements were being grouped into triads. Question (2): State Newland's 'Law of Octaves'. Answer: When elements are arranged in ascending order of their atomic weights, every eighth element had similar physical and chemical properties and resembled the first el ...
The Periodic Table
... become much less safe than today’s vehicles? – No, hydrogen fuel does not produce hot ash or radiant heat as gasoline, and hydrogen leaks disperse rapidly in the atmosphere resulting in less time to burn; Issues with tank material, strength, and ...
... become much less safe than today’s vehicles? – No, hydrogen fuel does not produce hot ash or radiant heat as gasoline, and hydrogen leaks disperse rapidly in the atmosphere resulting in less time to burn; Issues with tank material, strength, and ...
atomic - Sammons Sci
... React vigorously with most metal to form salts Have 7 electrons in outer shell Metalloids are along zigzag line, brittle metals with properties of both metals and ...
... React vigorously with most metal to form salts Have 7 electrons in outer shell Metalloids are along zigzag line, brittle metals with properties of both metals and ...
ionization energy
... lose these electrons to gain electron configurations that make them stable. The number of protons do not change therefore when a metallic atom loses an electron (negatively charge particle) it becomes positively charged because it now has less negative charges than it originally had. When they lose ...
... lose these electrons to gain electron configurations that make them stable. The number of protons do not change therefore when a metallic atom loses an electron (negatively charge particle) it becomes positively charged because it now has less negative charges than it originally had. When they lose ...
The Periodic Table
... Origins of the Periodic Table By the year 1700, only 13 elements had been identified Scientific discovery led to a higher rate of element discovery A logical organization of elements was needed for all the new elements ...
... Origins of the Periodic Table By the year 1700, only 13 elements had been identified Scientific discovery led to a higher rate of element discovery A logical organization of elements was needed for all the new elements ...
B - SchoolRack
... Which scientist proposed a model of the atom in which the individual atoms were thought of as positively charged spheres with negatively charged electrons orbiting in specific layers or shells? The atom absorbs or gives off specific amounts of energy when the electrons move from one shell to ...
... Which scientist proposed a model of the atom in which the individual atoms were thought of as positively charged spheres with negatively charged electrons orbiting in specific layers or shells? The atom absorbs or gives off specific amounts of energy when the electrons move from one shell to ...
chemical periodicity
... Listed 60+ elements in several vertical columns in order of their increasing atomic mass Noticed a regular recurrence of their physical and chemical properties ____________________ in the table because there were no known elements with the appropriate properties at that time 1871 - Predicted the phy ...
... Listed 60+ elements in several vertical columns in order of their increasing atomic mass Noticed a regular recurrence of their physical and chemical properties ____________________ in the table because there were no known elements with the appropriate properties at that time 1871 - Predicted the phy ...
AtomTest
... Labeling Information on the Periodic Table of Elements Select the pen tool from the box on the bottom left hand corner of this page and use it to draw a line from each label to its place on the periodic table. When you are done select automatic pointer and click the bottom right arrow to check ...
... Labeling Information on the Periodic Table of Elements Select the pen tool from the box on the bottom left hand corner of this page and use it to draw a line from each label to its place on the periodic table. When you are done select automatic pointer and click the bottom right arrow to check ...
2013 The Periodic Table
... 18 elements are called the noble gases and have eight valence electrons, except for helium which only has two. The noble gases with 8 valence electrons obey the octet rule and are ...
... 18 elements are called the noble gases and have eight valence electrons, except for helium which only has two. The noble gases with 8 valence electrons obey the octet rule and are ...
Atoms and periodic properties
... single capitalized letter for their symbol • The rest, that have permanent names have two letters. – the first is capitalized and the second is lower case – the lower case letter is either the second letter in the name, or the letter of a strong consonant heard when the name of the element is spoken ...
... single capitalized letter for their symbol • The rest, that have permanent names have two letters. – the first is capitalized and the second is lower case – the lower case letter is either the second letter in the name, or the letter of a strong consonant heard when the name of the element is spoken ...
Chapter 6 - HCC Learning Web
... • In general, the ionization energy increases as you go from the bottom to the top in a group. • In general, the ionization energy increases as you go from left to right across a period of elements. • The closer the electron to the nucleus, the more energy is required to remove the electron and thus ...
... • In general, the ionization energy increases as you go from the bottom to the top in a group. • In general, the ionization energy increases as you go from left to right across a period of elements. • The closer the electron to the nucleus, the more energy is required to remove the electron and thus ...
Introduction to Mendeleev*s Periodic Table of Elements
... • All the atoms in the same period have the same number of shells. • Same number of shells = same shielding, but the number of protons increase, therefore…. • Increasing nuclear charge …helps pull e- in tighter, therefore it is harder to remove. • So IE generally increases from left to right. ...
... • All the atoms in the same period have the same number of shells. • Same number of shells = same shielding, but the number of protons increase, therefore…. • Increasing nuclear charge …helps pull e- in tighter, therefore it is harder to remove. • So IE generally increases from left to right. ...
chem 1405 chapter -5
... These are the elements of the 8A group. They have completely filled sub shells in the valence shell. 3. Transition Metals ( d - block elements) They are the elements of the groups IB and 3B through 8B. All of them are metals with incompletely filled (n-1) d sub shells. Elements of 2B have completely ...
... These are the elements of the 8A group. They have completely filled sub shells in the valence shell. 3. Transition Metals ( d - block elements) They are the elements of the groups IB and 3B through 8B. All of them are metals with incompletely filled (n-1) d sub shells. Elements of 2B have completely ...
Chapter 6 - The Periodic Table
... S is the number of electrons blocking the valence shell electrons, the underlying noble gas electrons. Charge felt by 2s e- in Li Z* = 3 - 2 = 1 Be Z* = 4 - 2 = 2 B Z* = 5 - 2 = 3 and so on! ...
... S is the number of electrons blocking the valence shell electrons, the underlying noble gas electrons. Charge felt by 2s e- in Li Z* = 3 - 2 = 1 Be Z* = 4 - 2 = 2 B Z* = 5 - 2 = 3 and so on! ...
Group 3 element

Group 3 is a group of elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group. Scandium (Sc) and yttrium (Y) are always included, but the other two spaces are usually occupied by lanthanum (La) and actinium (Ac), or by lutetium (Lu) and lawrencium (Lr); less frequently, it is considered the group should be expanded to 32 elements (with all the lanthanides and actinides included) or contracted to contain only scandium and yttrium. The group itself has not acquired a trivial name; however, scandium, yttrium and the lanthanides are sometimes called rare earth metals.Three group 3 elements occur naturally, scandium, yttrium, and either lanthanum or lutetium. Lanthanum continues the trend started by two lighter members in general chemical behavior, while lutetium behaves more similarly to yttrium. This is in accordance with the trend for period 6 transition metals to behave more similarly to their upper periodic table neighbors. This trend is seen from hafnium, which is almost identical chemically to zirconium, to mercury, which is quite distant chemically from cadmium, but still shares with it almost equal atomic size and other similar properties. They all are silvery-white metals under standard conditions. The fourth element, either actinium or lawrencium, has only radioactive isotopes. Actinium, which occurs only in trace amounts, continues the trend in chemical behavior for metals that form tripositive ions with a noble gas configuration; synthetic lawrencium is calculated and partially shown to be more similar to lutetium and yttrium. So far, no experiments have been conducted to synthesize any element that could be the next group 3 element. Unbiunium (Ubu), which could be considered a group 3 element if preceded by lanthanum and actinium, might be synthesized in the near future, it being only three spaces away from the current heaviest element known, ununoctium.