* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download c1l2ch06
Survey
Document related concepts
Transcript
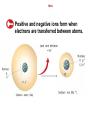
6.1 Searching For an Organizing Principle Searching For an Organizing Principle How did chemists begin to organize the known elements? 6.1 Searching For an Organizing Principle Certain chemical properties are repeating - reactivity with water (Na, K, etc.) - reactivity with other elements, etc. 6.1 Searching For an Organizing Principle Chlorine, bromine, and iodine have very similar chemical properties. 6.1 Mendeleev’s Periodic Table Mendeleev - Arranged elements by properties and atomic mass. - First example of a Periodic Table of the Elements. - Mendeleev's approach allowed him to successfully predict the masses and properties of elements that were not known at the time he developed his table. 6.1 Mendeleev’s Periodic Table An Early Version of Mendeleev’s Periodic Table The Modern Periodic Table Moseley - Mendeleev's arrangement was not completely uniform, some elements were apparently 'out of place'. Note the masses of Te and I in Mendeleev's table. - Moseley discovered that the nuclear charge increased for each increase of atomic mass. - This work provided experimental justification for the modern form of the Periodic Table. - This work also resulted in defining atomic number. The Periodic Law Moseley's refinement of Mendeleev's table gave us the modern statement of the Periodic Law. Periodic Table of the Elements - Arrangement of elements by atomic number so that elements with similar properties fall in the same column. Periodic Law - physical and chemical properties of the elements are periodic functions of their atomic numbers. There have been a number of additions to Mendeleev's table since he first proposed it, but the underlying principle that elements could be arranged by properties is still the same. 6.1 The Periodic Law In the modern periodic table, elements are arranged in order of increasing atomic number. 6.1 The Periodic Law The periodic law: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. • The properties of the elements within a period change as you move across a period from left to right. • The pattern of properties within a period repeats as you move from one period to the next. 6.1 Metals, Nonmetals, and Metalloids Metals, Nonmetals, and Metalloids What are three broad classes of elements? 6.1 Metals, Nonmetals, and Metalloids Three classes of elements are metals, nonmetals, and metalloids. Across a period, the properties of elements become less metallic and more nonmetallic. 6.1 Metals, Nonmetals, and Metalloids Metals, Metalloids, and Nonmetals in the Periodic Table 6.1 Metals, Nonmetals, and Metalloids Metals, Metalloids, and Nonmetals in the Periodic Table 6.1 Metals, Nonmetals, and Metalloids Metals, Metalloids, and Nonmetals in the Periodic Table 6.1 Metals, Nonmetals, and Metalloids Metals, Metalloids, and Nonmetals in the Periodic Table 6.1 Metals, Nonmetals, and Metalloids Metals Metals are good conductors of heat and electric current. • 80% of elements are metals. • Metals have a high luster, are ductile, and are malleable. 6.1 Metals, Nonmetals, and Metalloids Uses of Iron, Copper, and Aluminum 6.1 Metals, Nonmetals, and Metalloids Uses of Iron, Copper, and Aluminum 6.1 Metals, Nonmetals, and Metalloids Uses of Iron, Copper, and Aluminum 6.1 Metals, Nonmetals, and Metalloids Nonmetals In general, nonmetals are poor conductors of heat and electric current. • Most nonmetals are gases at room temperature. • A few nonmetals are solids, such as sulfur and phosphorus. • One nonmetal, bromine, is a dark-red liquid. 6.1 Metals, Nonmetals, and Metalloids Metalloids A metalloid generally has properties that are similar to those of metals and nonmetals. The behavior of a metalloid can be controlled by changing conditions. 6.1 Metals, Nonmetals, and Metalloids If a small amount of boron is mixed with silicon, the mixture is a good conductor of electric current. Silicon can be cut into wafers, and used to make computer chips. 6.2 Classifying the Elements A coin may contain much information in a small space—its value, the year it was minted, and its country of origin. Each square in a periodic table also contains information. You will learn what types of information are usually listed in a periodic table. 6.2 Squares in the Periodic Table Squares in the Periodic Table What type of information can be displayed in a periodic table? 6.2 Squares in the Periodic Table The periodic table displays the symbols and names of the elements, along with information about the structure of their atoms. 6.2 Squares in the Periodic Table The background colors in the squares are used to distinguish groups of elements. • The Group 1A elements are called alkali metals. • The Group 2A elements are called alkaline earth metals. • The nonmetals of Group 7A are called halogens. 6.2 Squares in the Periodic Table 6.2 Electron Configurations in Groups Electron Configurations in Groups How can elements be classified based on their electron configurations? 6.2 Electron Configurations in Groups Elements can be sorted into noble gases, representative elements, transition metals, or inner transition metals based on their electron configurations. 6.2 The blimp contains helium, one of the noble gases. Electron Configurations in Groups 6.2 Electron Configurations in Groups The Noble Gases The noble gases are the elements in Group 8A of the periodic table. The electron configurations for the first four noble gases in Group 8A are listed below. 6.2 Electron Configurations in Groups The Representative Elements Elements in groups 1A through 7A are often referred to as representative elements because they display a wide range of physical and chemical properties. • The s and p sublevels of the highest occupied energy level are not filled. • The group number equals the number of electrons in the highest occupied energy level. 6.2 Electron Configurations in Groups In atoms of the Group 1A elements below, there is only one electron in the highest occupied energy level. 6.2 Electron Configurations in Groups In atoms of the Group 4A elements below, there are four electrons in the highest occupied energy level. 6.2 Representative Elements Representative Elements 6.2 Representative Elements Representative Elements 6.2 Representative Elements Representative Elements 6.2 Representative Elements Representative Elements 6.2 Transition Elements Transition Elements There are two types of transition elements— transition metals and inner transition metals. They are classified based on their electron configurations. 6.2 Transition Elements In atoms of a transition metal, the highest occupied s sublevel and a nearby d sublevel contain electrons. In atoms of an inner transition metal, the highest occupied s sublevel and a nearby f sublevel generally contain electrons. 6.2 Blocks of Elements Transition Elements 6.3 Trends in Atomic Size Trends in Atomic Size What are the trends among the elements for atomic size? 6.3 Trends in Atomic Size The atomic radius is one half of the distance between the nuclei of two atoms of the same element when the atoms are joined. 6.3 Trends in Atomic Size Group and Periodic Trends in Atomic Size • In general, atomic size increases from top to bottom within a group and decreases from left to right across a period. 6.3 Trends in Atomic Size 6.3 Trends in Atomic Size Atomic radius Defined as one-half the distance between nuclei of identical atoms joined in a molecule. Across period - as Z increases, n remains the same. Increased attraction of electrons in same n. Remember n gives some indication of location of electrons from nucleus. Down group - Z increases, so does n. Electrons are farther away from positive nucleus, attractive force for valence electrons decreases. 6.3 Ions Some compounds are composed of particles called ions. • An ion is an atom or group of atoms that has a positive or negative charge. • A cation is an ion with a positive charge. • An anion is an ion with a negative charge. 6.3 Ions How do ions form? Ions 6.3 Ions Positive and negative ions form when electrons are transferred between atoms. 6.3 Ions Positive and negative ions form when electrons are transferred between atoms. 6.3 Trends in Ionization Energy The energy required to remove an electron from an atom is called ionization energy. • The energy required to remove the first electron from an atom is called the first ionization energy. • The energy required to remove an electron from an ion with a 1+ charge is called the second ionization energy. 6.3 Trends in Ionization Energy Group and Periodic Trends in Ionization Energy First ionization energy tends to decrease from top to bottom within a group and increase from left to right across a period. 6.3 Trends in Ionization Energy 6.3 Trends in Ionization Energy 6.3 Trends in Ionization Energy Ionization Energy (IE) Defined as the amount of energy necessary to remove electrons from an element in the gaseous state. First Ionization A + energy → A+ + eAcross period - increasing Z, increasing IE, e - held 'tightly' Down group - increasing Z, decreasing IE, e - less tightly held Higher Ionization Energies A + energy → Ab+ + beb - number of the ionization What is the reason for some of the other variations in ionization energies? Large jump between 1st and 2nd in Na Large jump between 2nd and 3rd in Mg Check out changes in electronic configurations. 6.3 Trends in Ionic Size Trends in Ionic Size During reactions between metals and nonmetals, metal atoms tend to lose electrons, and nonmetal atoms tend to gain electrons. The transfer has a predictable effect on the size of the ions that form. 6.3 Trends in Ionic Size Cations are always smaller than the atoms from which they form. Anions are always larger than the atoms from which they form. 6.3 Trends in Ionic Size Relative Sizes of Some Atoms and Ions 6.3 Size generally increases Trends in Ionic Size Trends in Ionic Size 6.3 Trends in Electronegativity Trends in Electronegativity Electronegativity is the ability of an atom of an element to attract electrons when the atom is in a compound. • In general, electronegativity values decrease from top to bottom within a group. For representative elements, the values tend to increase from left to right across a period. 6.3 Trends in Electronegativity Representative Elements in Groups 1A through 7A Electronegativity (Pauling Scale) Measure of the ability of an atom to attract electrons This does not necessarily mean anything about reactivity under a given set of conditions. F - has the highest Fr - has the lowest Electronegativity also gives an indication of the tendency of atoms to form cations or anions. High electronegativity usually means an atom will favor forming anions. Atoms tend to form ions in an effort to attain a valence electronic configuration that is isoelectronic with a Noble gas. 6.3 Summary of Trends Summary of Trends What is the underlying cause of periodic trends? 6.3 Summary of Trends The trends that exist among these properties can be explained by variations in atomic structure. 6.3 Summary of Trends Increases Decreases Size Electronegativity Ionic Atomic Nuclear Shielding Ionization of size cations Size anions Charge energy Increases Decreases Constant