Chemical Periodicity
... • Heath Chemistry: Experiments and Principles, Chapter 7 Order Among Atomsdels of Atomic ...
... • Heath Chemistry: Experiments and Principles, Chapter 7 Order Among Atomsdels of Atomic ...
Chapter 7 Periodic Properties of the Elements
... • In an isoelectronic series, ions have the same number of electrons. • Ionic size decreases with an increasing ...
... • In an isoelectronic series, ions have the same number of electrons. • Ionic size decreases with an increasing ...
Periodic Trends Studyguide with Questions and Answers
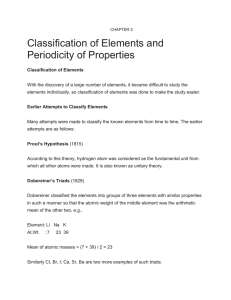
... . Chemical properties of the elements are periodic function of their atomic numbers . The elements on the Periodic Table are categorized as metals, nonmetals, or metalloids . More than two thirds of the elements are metals . The Periodic Table contains elements that are in all three phases (solid, l ...
... . Chemical properties of the elements are periodic function of their atomic numbers . The elements on the Periodic Table are categorized as metals, nonmetals, or metalloids . More than two thirds of the elements are metals . The Periodic Table contains elements that are in all three phases (solid, l ...
Chemistry: Matter and Change
... Development of the Periodic Table (cont.) • Meyer and Mendeleev both demonstrated a connection between atomic mass and elemental properties. • Moseley rearranged the table by increasing atomic number, and resulted in a clear periodic pattern. • Periodic repetition of chemical and physical propertie ...
... Development of the Periodic Table (cont.) • Meyer and Mendeleev both demonstrated a connection between atomic mass and elemental properties. • Moseley rearranged the table by increasing atomic number, and resulted in a clear periodic pattern. • Periodic repetition of chemical and physical propertie ...
Protons, Valence Electrons, and the Periodic Table
... • Atoms are the simplest form of an element that cannot be broken down chemically. • They maintain the element’s properties. • Each element is an atom that has the same number of protons – – i.e. the same atomic number on the periodic table of elements. ...
... • Atoms are the simplest form of an element that cannot be broken down chemically. • They maintain the element’s properties. • Each element is an atom that has the same number of protons – – i.e. the same atomic number on the periodic table of elements. ...
Honors Chemistry Periodic Table Notes Antoine Lavoisier (1700`s
... Cations have ______ electrons to become __________ charged– they become smaller than their ___________ atom. Electrons lost are __________ electrons. This can leave an empty outer orbital resulting in a smaller ________. Electrostatic repulsion between remaining electrons decreases, so remaining ele ...
... Cations have ______ electrons to become __________ charged– they become smaller than their ___________ atom. Electrons lost are __________ electrons. This can leave an empty outer orbital resulting in a smaller ________. Electrostatic repulsion between remaining electrons decreases, so remaining ele ...
Isotopes and the Electron Configuration of the Blocks in the Periodic
... ends the Table in this its version) became shifted for 4 positions to right. Therefore, a question rose: how to locate these 37 elements in the new version of the Table so that they would completely satisfy all the rules of the electron configuration of the blocks? First, we added 2 elements to bloc ...
... ends the Table in this its version) became shifted for 4 positions to right. Therefore, a question rose: how to locate these 37 elements in the new version of the Table so that they would completely satisfy all the rules of the electron configuration of the blocks? First, we added 2 elements to bloc ...
chemistry 1000 - U of L Class Index
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Z eff ). The fo ...
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Z eff ). The fo ...
II. Ch. 5.2: Electron Configuration and the Periodic Table
... When he arranged elements by increasing ______________ ________________, he noticed that similar elements occurred at regular intervals. ...
... When he arranged elements by increasing ______________ ________________, he noticed that similar elements occurred at regular intervals. ...
Electronegativity Periodic Trend
... button above the miniature periodic table. Now you can choose which elements, groups and/or periods you would like to graph. Select any period (excluding 1, 6 and 7) on the periodic table by clicking on the period number on the left. In the Graph window to the right, click on “X” or “Y” to choose th ...
... button above the miniature periodic table. Now you can choose which elements, groups and/or periods you would like to graph. Select any period (excluding 1, 6 and 7) on the periodic table by clicking on the period number on the left. In the Graph window to the right, click on “X” or “Y” to choose th ...
Periodic Table of the Elements
... Groups of the Periodic Table • Mendeleev put elements in the same group that had the same number of valence electrons – These valence electrons are lost or gained during chemical reactions – Elements in Period 1 and the first half of Period 2 gain or lose electrons in order to become like HELIUM wi ...
... Groups of the Periodic Table • Mendeleev put elements in the same group that had the same number of valence electrons – These valence electrons are lost or gained during chemical reactions – Elements in Period 1 and the first half of Period 2 gain or lose electrons in order to become like HELIUM wi ...
Mendeleef`s Periodic Table
... Anomalous behaviour of the first element of a group. The first element of a group differs considerably from its congeners (i.e., the rest of the elements of its group). This is due to (i) small size (ii) high electronegativity and (iii) non availability of d·orbitals for bonding. Anomalous behaviour ...
... Anomalous behaviour of the first element of a group. The first element of a group differs considerably from its congeners (i.e., the rest of the elements of its group). This is due to (i) small size (ii) high electronegativity and (iii) non availability of d·orbitals for bonding. Anomalous behaviour ...
Ri Christmas Lectures 2012: The Modern Alchemist
... Protons, Neutrons, and Electrons: Protons carry a relative positive charge, and have a relative mass of 1. The number of Protons in the nucleus determines which Element the atom is, and it is the number of Protons which gives an element its 'Atomic Number'. Neutrons carry no charge, and have a relat ...
... Protons, Neutrons, and Electrons: Protons carry a relative positive charge, and have a relative mass of 1. The number of Protons in the nucleus determines which Element the atom is, and it is the number of Protons which gives an element its 'Atomic Number'. Neutrons carry no charge, and have a relat ...
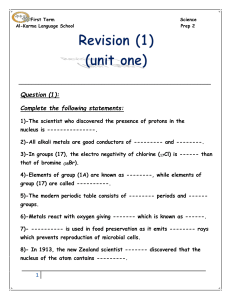
First Term Science Al-Karma Language School Prep 2 Question (1
... 12)-Sodium and potassium are kept under the surface of --------- to prevent them from the reaction with ----------. 13)-Each period in the modern periodic table starts with ------- and ends with ---------. 14)-The positive ion carries a number of ---------- charges equals to the number -------- elec ...
... 12)-Sodium and potassium are kept under the surface of --------- to prevent them from the reaction with ----------. 13)-Each period in the modern periodic table starts with ------- and ends with ---------. 14)-The positive ion carries a number of ---------- charges equals to the number -------- elec ...
Chapter 4 - ETSU.edu
... atomic numbers for chemical elements. He discovered isotopes, explaining how atomic mass did not order the elements appropriately. Moseley was the catalyst for the periodic law, a rule stating that the properties of elements are a periodic function of their atomic numbers. Using the atomic numbers, ...
... atomic numbers for chemical elements. He discovered isotopes, explaining how atomic mass did not order the elements appropriately. Moseley was the catalyst for the periodic law, a rule stating that the properties of elements are a periodic function of their atomic numbers. Using the atomic numbers, ...
periodic table
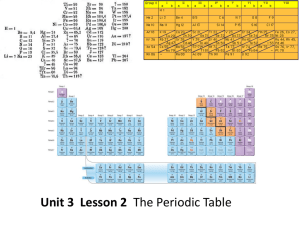
... How are the elements arranged on the periodic table? • Each vertical column of elements on the periodic table is called a group, or family. There are 18 groups. • Elements in a group are similar because their atoms have the same number of valence electrons. • Valence electrons are the electrons foun ...
... How are the elements arranged on the periodic table? • Each vertical column of elements on the periodic table is called a group, or family. There are 18 groups. • Elements in a group are similar because their atoms have the same number of valence electrons. • Valence electrons are the electrons foun ...
What is the PERIODIC TABLE?
... linear accelerator at the GSI Helmholtz Center for Heavy Ion Research in Germany. It created the calcium-ions used in new tests that produced element 117. For now, number 117 is the most massive element confirmed to exist! Success Criteria: Can I recognize that all matter consists of atoms? (SPI0807 ...
... linear accelerator at the GSI Helmholtz Center for Heavy Ion Research in Germany. It created the calcium-ions used in new tests that produced element 117. For now, number 117 is the most massive element confirmed to exist! Success Criteria: Can I recognize that all matter consists of atoms? (SPI0807 ...
Unit 3 Lesson 2 The Periodic Table Essential Question: How are
... Get Organized! What are elements? ...
... Get Organized! What are elements? ...
C11 Periodic Table Trends Powerpoint
... remove one mole of electrons from one mole of isolated gaseous atoms or ions. You may think of ionization energy as a measure of the difficulty of removing electron or the strength by which an electron is bound. The higher the ionization energy, the more difficult it is to remove an electron. Theref ...
... remove one mole of electrons from one mole of isolated gaseous atoms or ions. You may think of ionization energy as a measure of the difficulty of removing electron or the strength by which an electron is bound. The higher the ionization energy, the more difficult it is to remove an electron. Theref ...
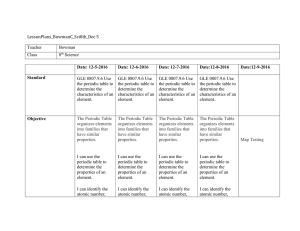
LessonPlans_BowmanC_Sci8th_Dec 5 Teacher Bowman Class 8th
... (Then First Letter of Mendeleev Video Last Name) IV. Count the Compare Days of Table (How many the Week, Groups, How many Calendars, Cards Periods) II. Periodic Table with A Video Series about Mendeleev: Mendeleev Video III. Review Atomic Symbols, Atomic Mass, Atomic Number IV. We Do: Periodic Tabl ...
... (Then First Letter of Mendeleev Video Last Name) IV. Count the Compare Days of Table (How many the Week, Groups, How many Calendars, Cards Periods) II. Periodic Table with A Video Series about Mendeleev: Mendeleev Video III. Review Atomic Symbols, Atomic Mass, Atomic Number IV. We Do: Periodic Tabl ...
ppt - Ms Jilesen
... How many +1 would you need to balance the -2 to zero? Since you need 2 atoms of the 1+ to balance the 2- to zero the resulting compound would be H2O In other words: to combine H with O, you MUST have 2 H to balance the oxidation numbers to zero 2+ and 2- = ZERO ...
... How many +1 would you need to balance the -2 to zero? Since you need 2 atoms of the 1+ to balance the 2- to zero the resulting compound would be H2O In other words: to combine H with O, you MUST have 2 H to balance the oxidation numbers to zero 2+ and 2- = ZERO ...
Review for the Physical Science Final
... extremely small. Ordinary-sized objects are made up of very large numbers of atoms. The mass number of an atom is the sum of the atom’s neutrons and protons. ...
... extremely small. Ordinary-sized objects are made up of very large numbers of atoms. The mass number of an atom is the sum of the atom’s neutrons and protons. ...
Document
... which all matter...is of one and the same kind; this matter being the substance from which all the chemical elements are built up." • “I can see no escape from the conclusion that [cathode rays] are charges of electricity carried by particles of matter.” but... ...
... which all matter...is of one and the same kind; this matter being the substance from which all the chemical elements are built up." • “I can see no escape from the conclusion that [cathode rays] are charges of electricity carried by particles of matter.” but... ...
Unit 13
... C As you learned in Unit 5, an atom can gain or lose electrons to form an ion. C When an atom loses electrons it becomes smaller. C Loss of electrons not only vacates the atom’s largest orbitals, it also reduces the repulsive force between the remaining electrons, allowing them to be pulled closed t ...
... C As you learned in Unit 5, an atom can gain or lose electrons to form an ion. C When an atom loses electrons it becomes smaller. C Loss of electrons not only vacates the atom’s largest orbitals, it also reduces the repulsive force between the remaining electrons, allowing them to be pulled closed t ...
Chapter 3 Physical Science - St. Pius X Classical Academy
... in an unstable nucleus during each form of radioactive decay? ...
... in an unstable nucleus during each form of radioactive decay? ...