Periodic data and trends assignment
... 4a) What is happening to the number of protons and the number of energy levels as you move across the periodic table from left to right? How and why does this affect atomic radius. The number of protons is increasing but the energy level remains the same. As more and more protons are added, the ioni ...
... 4a) What is happening to the number of protons and the number of energy levels as you move across the periodic table from left to right? How and why does this affect atomic radius. The number of protons is increasing but the energy level remains the same. As more and more protons are added, the ioni ...
Periodic Classification of Elements
... always regular from one to its next. It was believed that a more fundamental property than atomic mass could explain periodic properties in a better manner. It was Henry Moseley who demonstrated that atomic number of an element could explain periodic properties in a better way than atomic mass of an ...
... always regular from one to its next. It was believed that a more fundamental property than atomic mass could explain periodic properties in a better manner. It was Henry Moseley who demonstrated that atomic number of an element could explain periodic properties in a better way than atomic mass of an ...
Atoms and the Periodic Table Subatomic Particles
... Atomic Weight (Mass) • Atomic Weight (Mass) - The average mass of all of the isotopes of an element that occur in nature. • Carbon-12 is assigned a mass of exactly 12 amu. ...
... Atomic Weight (Mass) • Atomic Weight (Mass) - The average mass of all of the isotopes of an element that occur in nature. • Carbon-12 is assigned a mass of exactly 12 amu. ...
File - pic sciences
... In a group the electro negativity ---------------- from top to bottom. Addition of hydrogen to a given compound is called ---------------Elements from atomic Number 58 to 71 are known as ---------------The ---------------- attempt of classification of elements are made by Dobereiner. In ------------ ...
... In a group the electro negativity ---------------- from top to bottom. Addition of hydrogen to a given compound is called ---------------Elements from atomic Number 58 to 71 are known as ---------------The ---------------- attempt of classification of elements are made by Dobereiner. In ------------ ...
Chapter 7. Periodic Properties of the Elements
... Elements in same column contain same number of outer-shell electrons or valence electrons Arrange elements to reflect trends in chemical and physical properties Periodic table arises from the periodic patterns in electronic configurations of elements • elements in same column contain same number of ...
... Elements in same column contain same number of outer-shell electrons or valence electrons Arrange elements to reflect trends in chemical and physical properties Periodic table arises from the periodic patterns in electronic configurations of elements • elements in same column contain same number of ...
The Periodic Table
... USING THE PERIODIC TABLE Rows are called periods. Columns are called groups. ...
... USING THE PERIODIC TABLE Rows are called periods. Columns are called groups. ...
Introductory Chemistry: Concepts & Connections 4th Edition
... • The periodic law states that the properties of elements recur in a repeating pattern when arranged according to increasing atomic number. • With the introduction of the concept of electron energy levels by Niels Bohr, the periodic table ...
... • The periodic law states that the properties of elements recur in a repeating pattern when arranged according to increasing atomic number. • With the introduction of the concept of electron energy levels by Niels Bohr, the periodic table ...
Periodic Table
... • The periodic law states that the properties of elements recur in a repeating pattern when arranged according to increasing atomic number. • With the introduction of the concept of electron energy levels by Niels Bohr, the periodic table ...
... • The periodic law states that the properties of elements recur in a repeating pattern when arranged according to increasing atomic number. • With the introduction of the concept of electron energy levels by Niels Bohr, the periodic table ...
8th Grade Chap 4 Study Guide Answer Section
... a. is a mixture of a metal with at least one c. can be pulled out, or drawn, into a long other element. wire. b. can be hammered or rolled into flat sheets d. can transfer heat or electricity to another and other shapes. material. 22. The two most common alkaline earth metals are a. iron and silver. ...
... a. is a mixture of a metal with at least one c. can be pulled out, or drawn, into a long other element. wire. b. can be hammered or rolled into flat sheets d. can transfer heat or electricity to another and other shapes. material. 22. The two most common alkaline earth metals are a. iron and silver. ...
Document
... • The elements were first organized by increasing atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physica ...
... • The elements were first organized by increasing atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physica ...
Chapter 6
... 61. Which group of elements in the periodic table is known as the alkali metals? 62. Which group in the periodic table is known as the noble gases? 63. An element has an atomic number of 80. How many protons and electrons are in an atom of the element? 64. About what percent of elements is classifie ...
... 61. Which group of elements in the periodic table is known as the alkali metals? 62. Which group in the periodic table is known as the noble gases? 63. An element has an atomic number of 80. How many protons and electrons are in an atom of the element? 64. About what percent of elements is classifie ...
Mendeleev`s Periodic Table - Scotch Plains
... into three large groups based on their properties. Metals are good conductors and many are ductile and malleable. Nonmetals are mostly gases whose properties are opposite to those of metals. Metalloids can behave like metals or nonmetals, depending on the conditions. After reading Lesson 6.1, answer ...
... into three large groups based on their properties. Metals are good conductors and many are ductile and malleable. Nonmetals are mostly gases whose properties are opposite to those of metals. Metalloids can behave like metals or nonmetals, depending on the conditions. After reading Lesson 6.1, answer ...
Chapter 6 Periodic Table Lecture Notes
... • Columns of elements are called groups. • Rows of elements are called periods. • Elements in groups 1,2, and 13-18 possess a wide variety of chemical and physical properties and are called the representative elements. • Elements in groups 3-12 are known as the transition metals. ...
... • Columns of elements are called groups. • Rows of elements are called periods. • Elements in groups 1,2, and 13-18 possess a wide variety of chemical and physical properties and are called the representative elements. • Elements in groups 3-12 are known as the transition metals. ...
The Periodic Law Notes (Chapter 5) – Part 2
... 3. Group trend – ionization energy increases as you move up a group (or decreases as you move down a group). In general, as you do down a group the ionization energy decreases because the size of the atom is increasing and the outermost electrons are further from the nucleus. 1. Which atom has the h ...
... 3. Group trend – ionization energy increases as you move up a group (or decreases as you move down a group). In general, as you do down a group the ionization energy decreases because the size of the atom is increasing and the outermost electrons are further from the nucleus. 1. Which atom has the h ...
Chapter 5- The Periodic Law
... 1. Hydrogen does not share the same properties as the elements in Group 1 2. Helium possesses special chemical stability because of its filled outer shell E. The d-Block Elements: Groups 3-12 ...
... 1. Hydrogen does not share the same properties as the elements in Group 1 2. Helium possesses special chemical stability because of its filled outer shell E. The d-Block Elements: Groups 3-12 ...
Electronegativity - Malibu High School
... 1. How is atomic number arranged on the periodic table? 2. How is atomic mass arranged on the periodic table? ...
... 1. How is atomic number arranged on the periodic table? 2. How is atomic mass arranged on the periodic table? ...
Coloring the Periodic Table - Families
... scientist born in Tobolsk, Siberia in 1834, is known as the father of the periodic table of the elements. The periodic table of the elements is an important tool used by students and chemists around the world to help them understand and simplify the often complex world of chemical reactions. ...
... scientist born in Tobolsk, Siberia in 1834, is known as the father of the periodic table of the elements. The periodic table of the elements is an important tool used by students and chemists around the world to help them understand and simplify the often complex world of chemical reactions. ...
The Periodic Table & Formation of Ions
... Increases from left to right across a period Decreases from top to bottom in a group ...
... Increases from left to right across a period Decreases from top to bottom in a group ...
PeriodicTrends
... • SC4.Students will use the organization of the Periodic Table to predict properties of elements. • SC4a. Use the Periodic Table to predict periodic trends including atomic radii, ionic radii, ionization energy, and electronegativity of various elements. ...
... • SC4.Students will use the organization of the Periodic Table to predict properties of elements. • SC4a. Use the Periodic Table to predict periodic trends including atomic radii, ionic radii, ionization energy, and electronegativity of various elements. ...
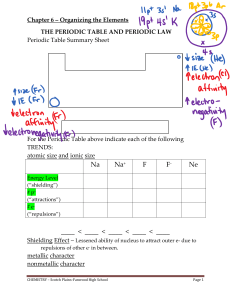
The Periodic Table and Periodic Law
... configuration of iron (a transition element) is [Ar]4s 23d 6, which has its valence electrons in orbits of both the third and fourth energy levels. ...
... configuration of iron (a transition element) is [Ar]4s 23d 6, which has its valence electrons in orbits of both the third and fourth energy levels. ...
File
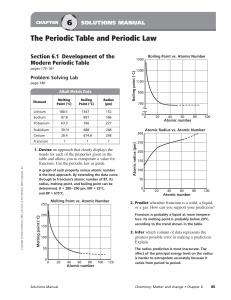
... The ionization energies for element X are listed in the table above. On the basis of the data, element X is most likely to ...
... The ionization energies for element X are listed in the table above. On the basis of the data, element X is most likely to ...
File - CCHS Chemistry
... It is sometimes possible to force the outer level of an elem in 3rd or higher period to hold more than 8 e-’s ◦ - Extended Octet Noble gas comps are formed this way ...
... It is sometimes possible to force the outer level of an elem in 3rd or higher period to hold more than 8 e-’s ◦ - Extended Octet Noble gas comps are formed this way ...
d) Ramsay. The idea of arranging the elements in the periodic table
... Periods with occupied f sublevels a) have only Group 1 and 2 elements. c) have 32 groups. ...
... Periods with occupied f sublevels a) have only Group 1 and 2 elements. c) have 32 groups. ...