A scientist named Henry Mosley developed the modern period table
... A scientist named Henry Mosley developed the modern period table. Mosley discovered if the elements were arranged a certain way, predictions could be made about the structure and properties of elements. Today we will investigate these patterns and discover that the periodic table is a useful tool wh ...
... A scientist named Henry Mosley developed the modern period table. Mosley discovered if the elements were arranged a certain way, predictions could be made about the structure and properties of elements. Today we will investigate these patterns and discover that the periodic table is a useful tool wh ...
Atomic Structure and The Periodic Table
... 1. How can the periodic table be used to find information about the element? 2. How does the organization of the periodic table reflect atomic structure? ...
... 1. How can the periodic table be used to find information about the element? 2. How does the organization of the periodic table reflect atomic structure? ...
Chemistry Midterm Exam Review Sheet
... 63. If the electronegativity difference between 2 atoms is greater than 1.70, a __________________ bond will form. 64. If the electronegativity difference between 2 atoms is less than 1.70, a ____________________ bond will form. ...
... 63. If the electronegativity difference between 2 atoms is greater than 1.70, a __________________ bond will form. 64. If the electronegativity difference between 2 atoms is less than 1.70, a ____________________ bond will form. ...
CP CHEMISTRY STUDY GUIDE
... Correctly identify an element’s common oxidation state (charge of an ion) based on its position on the Periodic Table, and explain the difference in the oxidation numbers of metals and nonmetals in terms of atomic structure and position on the Periodic Table. ...
... Correctly identify an element’s common oxidation state (charge of an ion) based on its position on the Periodic Table, and explain the difference in the oxidation numbers of metals and nonmetals in terms of atomic structure and position on the Periodic Table. ...
4.3 Exploring the Modern Periodic Table
... a) How is the electron arrangement similar for these two elements? (Hint: look at the outer shell) b) The elements Br and I are also in the second last column of the periodic table. How many electrons do you think would be in their outer electron shell? 8. Elements in the same column tend to form si ...
... a) How is the electron arrangement similar for these two elements? (Hint: look at the outer shell) b) The elements Br and I are also in the second last column of the periodic table. How many electrons do you think would be in their outer electron shell? 8. Elements in the same column tend to form si ...
trend lab
... Purpose: You will arrange the code elements in groups 1&2 (s block) and 13-18 ( the p block) (the entire d and f block is not present )according to atomic number, symbol, oxidation number, atomic radii, and electronegativity. Materials: Scissors, glue, tables Procedure: 1. Cut out all the blocks of ...
... Purpose: You will arrange the code elements in groups 1&2 (s block) and 13-18 ( the p block) (the entire d and f block is not present )according to atomic number, symbol, oxidation number, atomic radii, and electronegativity. Materials: Scissors, glue, tables Procedure: 1. Cut out all the blocks of ...
Reinforcing Key Concepts
... radioactivity by the time it takes for one-half of a sample of atoms to change identity. For example, lead-214 has a half-life of 27 minutes. If you started with 500 grams of this isotope, how many grams would you have after 54 minutes? ...
... radioactivity by the time it takes for one-half of a sample of atoms to change identity. For example, lead-214 has a half-life of 27 minutes. If you started with 500 grams of this isotope, how many grams would you have after 54 minutes? ...
NAME - Cobb Learning
... In 1870, Dmitri Mendeleev first proposed a new way of studying and organizing the then known 63 elements. The modern form of the table has been modified and improved many times since Mendeleev’s tables. Pioneers like Moseley (1913) and Seaborg (1941) have made the properties of the elements much sim ...
... In 1870, Dmitri Mendeleev first proposed a new way of studying and organizing the then known 63 elements. The modern form of the table has been modified and improved many times since Mendeleev’s tables. Pioneers like Moseley (1913) and Seaborg (1941) have made the properties of the elements much sim ...
Periodic Table of Elements
... The protons and neutrons are in the center of the atom which is called the nucleus The electrons are found outside of the nucleus Protons have a positive charge Neutrons have a neutral charge Electrons have a negative charge ...
... The protons and neutrons are in the center of the atom which is called the nucleus The electrons are found outside of the nucleus Protons have a positive charge Neutrons have a neutral charge Electrons have a negative charge ...
Chapter 15 – The Periodic Table
... elements in order of increasing atomic weights. Proposed the Law of Octaves. Meyer (1869) Compiled a periodic table with elements arranged by atomic weight. Elements with similar properties arranged in columns. Mendeleev (1869) Created a periodic with elements arranged by atomic weight. Elements ...
... elements in order of increasing atomic weights. Proposed the Law of Octaves. Meyer (1869) Compiled a periodic table with elements arranged by atomic weight. Elements with similar properties arranged in columns. Mendeleev (1869) Created a periodic with elements arranged by atomic weight. Elements ...
Periodic Table
... Elements in the same group have similar physical and chemical properties Sometimes groups are called families ...
... Elements in the same group have similar physical and chemical properties Sometimes groups are called families ...
Document
... Each of the seven energy levels can have a maximum number of electrons. Energy level one can contain at most two electrons. Energy level two can contain at most eight electrons. ...
... Each of the seven energy levels can have a maximum number of electrons. Energy level one can contain at most two electrons. Energy level two can contain at most eight electrons. ...
Name Period
... 6. A(n) _______________ transmits heat and electricity easily. 7. A material that is _________________ can be drawn into a wire. 8. ____________________ is the ease and speed with which an element combines with other elements and compounds. 9. A(n) ______________________ is a mixture of metals. ...
... 6. A(n) _______________ transmits heat and electricity easily. 7. A material that is _________________ can be drawn into a wire. 8. ____________________ is the ease and speed with which an element combines with other elements and compounds. 9. A(n) ______________________ is a mixture of metals. ...
Atoms, elements and compounds
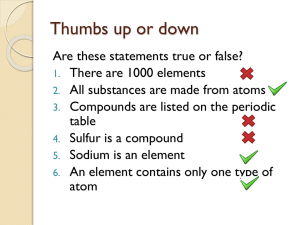
... All pupils will be able to (Baseline): Recognise that elements are made from only one type of particle known as an atom, which is the smallest part of an element. Most pupils will be able to (Further): Identify the first 20 elements in the periodic table and the elements in Groups 1 and 7 by their n ...
... All pupils will be able to (Baseline): Recognise that elements are made from only one type of particle known as an atom, which is the smallest part of an element. Most pupils will be able to (Further): Identify the first 20 elements in the periodic table and the elements in Groups 1 and 7 by their n ...
Atoms and the
... The complete symbol for an atom or ion consists of the elemental symbol surrounded by subscripts and superscripts. a) The leading superscript (upper left) is the mass number. This is also the number of nucleons; a nucleon is a proton or a neutron. b) The leading subscript (lower left) is the atomic ...
... The complete symbol for an atom or ion consists of the elemental symbol surrounded by subscripts and superscripts. a) The leading superscript (upper left) is the mass number. This is also the number of nucleons; a nucleon is a proton or a neutron. b) The leading subscript (lower left) is the atomic ...
Atoms and the
... The complete symbol for an atom or ion consists of the elemental symbol surrounded by subscripts and superscripts. a) The leading superscript (upper left) is the mass number. This is also the number of nucleons; a nucleon is a proton or a neutron. b) The leading subscript (lower left) is the atomic ...
... The complete symbol for an atom or ion consists of the elemental symbol surrounded by subscripts and superscripts. a) The leading superscript (upper left) is the mass number. This is also the number of nucleons; a nucleon is a proton or a neutron. b) The leading subscript (lower left) is the atomic ...
Int. Sci. 9 Modern Periodic Table Powerpoint
... – atoms of the same element so they have the same ATOMIC # (protons) & different MASS # (neutrons) There is not just 1 “regular” atom which you see on the Periodic Table The symbols on the Periodic Table represent “average” atoms of elements – A sample of an element found in nature contains a mi ...
... – atoms of the same element so they have the same ATOMIC # (protons) & different MASS # (neutrons) There is not just 1 “regular” atom which you see on the Periodic Table The symbols on the Periodic Table represent “average” atoms of elements – A sample of an element found in nature contains a mi ...
Reactivity of Atoms Based on Their Placement in The Periodic Table
... Elements are also classified based on whether the occur naturally or not Elements that do not occur naturally have an atomic number greater than 92 Check them out.... ...
... Elements are also classified based on whether the occur naturally or not Elements that do not occur naturally have an atomic number greater than 92 Check them out.... ...
File
... 5. Which of the following elements that have similar physical and chemical properties: a. K, Mn, Ge, Br b. Be, Mg, Sr, Ra c. B, Br, Ba, Bi d. N, O, F, Ne Objective 2 1. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium. ...
... 5. Which of the following elements that have similar physical and chemical properties: a. K, Mn, Ge, Br b. Be, Mg, Sr, Ra c. B, Br, Ba, Bi d. N, O, F, Ne Objective 2 1. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium. ...
The Periodic Table
... Metal characteristics • Solids at STP (except mercury) • good conductors of electricity and heat – Mobile electrons ...
... Metal characteristics • Solids at STP (except mercury) • good conductors of electricity and heat – Mobile electrons ...
Port Said International Schools
... 11.All the following elements don’t react with dilute HCL except (Cu- Zn- S- C) 12.Elements which have atomic number( 2,8,16- 2,10,18- 3,11,19- 4,12,20) are called alkali metals. 13.Alkali metals have the following properties except (they have low density- they conduct electricity- they conduct heat ...
... 11.All the following elements don’t react with dilute HCL except (Cu- Zn- S- C) 12.Elements which have atomic number( 2,8,16- 2,10,18- 3,11,19- 4,12,20) are called alkali metals. 13.Alkali metals have the following properties except (they have low density- they conduct electricity- they conduct heat ...
The Periodic Table of Elements - PAMS-Doyle
... • Have 6 valence electrons in their outer shell • They share electrons when chemically combined • Oxygen is the most abundant element in the Earth’s crust and second most abundant element in the atmosphere • Oxygen is an extremely reactive element and combines with almost all other elements • Family ...
... • Have 6 valence electrons in their outer shell • They share electrons when chemically combined • Oxygen is the most abundant element in the Earth’s crust and second most abundant element in the atmosphere • Oxygen is an extremely reactive element and combines with almost all other elements • Family ...
Chapter 7:
... any group because it has properties of both metals and nonmetals. It behaves as a metal when it loses its electron. It behaves as a nonmetal when it gains an electron. The universe contains more than 90% hydrogen by mass. Hydrogen reacts violently with oxygen in the production of water. The main use ...
... any group because it has properties of both metals and nonmetals. It behaves as a metal when it loses its electron. It behaves as a nonmetal when it gains an electron. The universe contains more than 90% hydrogen by mass. Hydrogen reacts violently with oxygen in the production of water. The main use ...
Periodic Table Timeline
... earliest ideas of atoms, molecules, and chemical reactions marking the beginning of the history of modern chemistry. ...
... earliest ideas of atoms, molecules, and chemical reactions marking the beginning of the history of modern chemistry. ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.