What is the periodic table of elements - Net Start Class
... atom of an element in a period has the same number of energy levels. Lithium has two energy levels, so it occurs in period two. Each of the 18 columns is a group, or family, of elements. All of the elements in a group have many similar chemical properties. These similarities occur due to the element ...
... atom of an element in a period has the same number of energy levels. Lithium has two energy levels, so it occurs in period two. Each of the 18 columns is a group, or family, of elements. All of the elements in a group have many similar chemical properties. These similarities occur due to the element ...
Chapter 5
... • The elements in Group 2A are called alkaline earth metals • All alkaline earth metals have two valence electrons • They are harder than group 1A • Differences in reactivity among the alkaline earth metals are shown by the ways they react with water • Calcium, strontium and barium react easily with ...
... • The elements in Group 2A are called alkaline earth metals • All alkaline earth metals have two valence electrons • They are harder than group 1A • Differences in reactivity among the alkaline earth metals are shown by the ways they react with water • Calcium, strontium and barium react easily with ...
Periodic Table ppt
... properties and is used in the computer industry. It is one of the few elements that expand when frozen. Lead has long been used for plumbing and is also used to block radiation. Tin was once used to make cans because it is relatively stable -- unreactive. Aluminum has replaced the more expensive tin ...
... properties and is used in the computer industry. It is one of the few elements that expand when frozen. Lead has long been used for plumbing and is also used to block radiation. Tin was once used to make cans because it is relatively stable -- unreactive. Aluminum has replaced the more expensive tin ...
Elements and the Periodic Table Practice Test
... 9. There exists several _____________ of argon, such as Ar-36, Ar-38, and Ar-40. 10.Yet the mass for argon on the periodic table is 39.948 atomic mass units and not 36, 38, or 40. Explain why. ...
... 9. There exists several _____________ of argon, such as Ar-36, Ar-38, and Ar-40. 10.Yet the mass for argon on the periodic table is 39.948 atomic mass units and not 36, 38, or 40. Explain why. ...
Anticipation Guide for Chem Talk pg 370-373
... 3. Mendeleev used atomic mass to organize his periodic table. a. Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. b. Students know how to use the periodic table to identify metals, semimetals, non-metals, and halogens. ...
... 3. Mendeleev used atomic mass to organize his periodic table. a. Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. b. Students know how to use the periodic table to identify metals, semimetals, non-metals, and halogens. ...
alkaline earth metals
... Nonmetals and metalloids have a wide range of properties. • Nonmetals—on right side of PT and include elements with a wide range of properties: carbon, nitrogen, sulfur, also the Halogens such as chlorine and the noble gases such as neon • Metalloids—in between metals and nonmetals in the PT—have c ...
... Nonmetals and metalloids have a wide range of properties. • Nonmetals—on right side of PT and include elements with a wide range of properties: carbon, nitrogen, sulfur, also the Halogens such as chlorine and the noble gases such as neon • Metalloids—in between metals and nonmetals in the PT—have c ...
The periodic table as we have it today has not always
... •Solids lack luster •Many gases at room temperature ...
... •Solids lack luster •Many gases at room temperature ...
atomic number - Net Start Class
... conductors of heat and electricity. Non-metals are not ductile or malleable. Solid non-metals are brittle and break easily. They are dull. Many non-metals are gases. ...
... conductors of heat and electricity. Non-metals are not ductile or malleable. Solid non-metals are brittle and break easily. They are dull. Many non-metals are gases. ...
Brown, Le May, and Bursten: Chapter 2
... Some elements can form more than one compound when they react together (C & O: CO and CO2; N & O: N2O, NO, NO2, etc.). Dalton’s law predicted that the mass proportions should be proportional. Experiment confirmed this leading to this law. Law of multiple proportions: when two elements form more than ...
... Some elements can form more than one compound when they react together (C & O: CO and CO2; N & O: N2O, NO, NO2, etc.). Dalton’s law predicted that the mass proportions should be proportional. Experiment confirmed this leading to this law. Law of multiple proportions: when two elements form more than ...
Chapter 5
... • The elements in Group_______ are called the noble gases • Helium has _______ valence electrons • All other noble gases have ______ valence electrons • The noble gases are________ and________ and extremely_________ • All the noble gases except ______ are used in “neon” lights ...
... • The elements in Group_______ are called the noble gases • Helium has _______ valence electrons • All other noble gases have ______ valence electrons • The noble gases are________ and________ and extremely_________ • All the noble gases except ______ are used in “neon” lights ...
Atoms, Bonding, and the Periodic Table Electron Dot Diagrams
... Electron Dot Diagrams The valence electrons of an atom are shown as dots around the symbol of the element. Complete the electron dot diagram for neon. ...
... Electron Dot Diagrams The valence electrons of an atom are shown as dots around the symbol of the element. Complete the electron dot diagram for neon. ...
Elements and the Periodic Table Section One
... Family: elements in the same vertical column of the periodic table; also called group (pg. 82) Group: elements in the same vertical column of the periodic table; also called family (pg. 82) Period: a horizontal row of elements in the periodic table (pg. 83) Valence Electron: one of the electrons far ...
... Family: elements in the same vertical column of the periodic table; also called group (pg. 82) Group: elements in the same vertical column of the periodic table; also called family (pg. 82) Period: a horizontal row of elements in the periodic table (pg. 83) Valence Electron: one of the electrons far ...
Notes # ____ - The Periodic Table
... characteristics repeat in a regular pattern. The table is read in rows, called “periods” and columns, called “groups” or “families”. Periods are read from left to right and the atomic number increases by one for each element. One way to remember this is that a “period” is often found at the end of a ...
... characteristics repeat in a regular pattern. The table is read in rows, called “periods” and columns, called “groups” or “families”. Periods are read from left to right and the atomic number increases by one for each element. One way to remember this is that a “period” is often found at the end of a ...
Chapter 5
... 〉What happens to an atom that gains or loses electrons? 〉If an atom gains or loses electrons, it no longer has an equal number of electrons and protons. Because the charges do not cancel completely, the atom has a net electric charge. ...
... 〉What happens to an atom that gains or loses electrons? 〉If an atom gains or loses electrons, it no longer has an equal number of electrons and protons. Because the charges do not cancel completely, the atom has a net electric charge. ...
Science Review Sheet: Periodic Table Test Name: __________
... 9. What are the elements in group 8a called? What makes them unique compared to other elements? ...
... 9. What are the elements in group 8a called? What makes them unique compared to other elements? ...
The Periodic Table
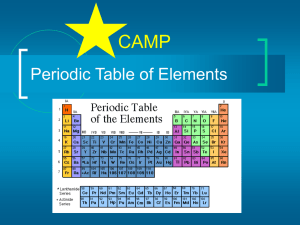
... of the element, the atomic number, and the atomic mass are printed. Some periodic tables have even more information than that ...
... of the element, the atomic number, and the atomic mass are printed. Some periodic tables have even more information than that ...
Powerpoint - Valence Electrons
... Br is a liquid and I is a solid F is the most reactive and I is the least Have a -1 ion charge ...
... Br is a liquid and I is a solid F is the most reactive and I is the least Have a -1 ion charge ...
Notes Chapter 5 “The Periodic Law” Section 1 Dmitri Mendeleev
... in s1, not found in nature as free elements. Always found in compounds. React strongly with water. Soft silvery appearance can be cut with a knife, will have a +1 charge). Group 2( alkaline earth metals, end in s2, harder, denser, and stronger than group 1, not as reactive as group 1, will have a +2 ...
... in s1, not found in nature as free elements. Always found in compounds. React strongly with water. Soft silvery appearance can be cut with a knife, will have a +1 charge). Group 2( alkaline earth metals, end in s2, harder, denser, and stronger than group 1, not as reactive as group 1, will have a +2 ...
CHEMISTRY NOTES 9.1.1 ATOMS, ELEMENTS, PERIODIC TABLE
... Malleable (can be shaped), fusible (can be fused or melted), ductile (can be formed into wire) Lose the electrons in their outer shell; form metallic and ionic bonds; transmit heat & electricity easily because of flow of electrons; form metallic bonds between atoms of the same element All metals are ...
... Malleable (can be shaped), fusible (can be fused or melted), ductile (can be formed into wire) Lose the electrons in their outer shell; form metallic and ionic bonds; transmit heat & electricity easily because of flow of electrons; form metallic bonds between atoms of the same element All metals are ...
Periodic Table Cloze - Science
... _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the Calcium: an element on the periodic table with atomic number 20. ...
... _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the Calcium: an element on the periodic table with atomic number 20. ...
9The-Periodic-table1 (3).pptx
... ! The electrons in the outer most energy level of the atom ! Allow atoms to form chemical bonds with other atoms ! All elements in the same group ( column) have similar chemical properties ! ...
... ! The electrons in the outer most energy level of the atom ! Allow atoms to form chemical bonds with other atoms ! All elements in the same group ( column) have similar chemical properties ! ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.