Name
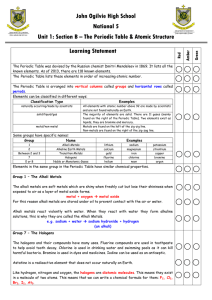
... The chemical makeup of the alien planet’s oceans seems to be about the same as Earth’s ocean. When sea water is distilled, the liquid has been shown to have molecules consisting of two atoms pfsst (Pf) and one atom of nuutye (Nu). The solid left behind after the distillation consists of a crystal ma ...
... The chemical makeup of the alien planet’s oceans seems to be about the same as Earth’s ocean. When sea water is distilled, the liquid has been shown to have molecules consisting of two atoms pfsst (Pf) and one atom of nuutye (Nu). The solid left behind after the distillation consists of a crystal ma ...
File
... Circle or highlight the correct word(s) in the brackets [ ] to complete the following sentences. [Nitrogen, Oxygen, Argon] has a full valence shell and is a noble gas. Noble gases are [inert, very reactive, only react with certain elements]. [Potassium, Calcium, Sulfur, Neon] has properties most sim ...
... Circle or highlight the correct word(s) in the brackets [ ] to complete the following sentences. [Nitrogen, Oxygen, Argon] has a full valence shell and is a noble gas. Noble gases are [inert, very reactive, only react with certain elements]. [Potassium, Calcium, Sulfur, Neon] has properties most sim ...
assignment-07-a3
... involve removing an electron from a stable inert gas-like electron configuration. This would be much larger than the second ionization energy for Be, where the second-ionization step results in the formation of an inert gas like electron configuration.) 14- For each of the following pairs, which ele ...
... involve removing an electron from a stable inert gas-like electron configuration. This would be much larger than the second ionization energy for Be, where the second-ionization step results in the formation of an inert gas like electron configuration.) 14- For each of the following pairs, which ele ...
Activity 16 Elements and the Periodic Table
... Do you see another of the patterns that Mendeleev and other scientists recognized? ___________ Neutral atoms contain equal numbers of protons (+) and electrons (-). Because of this, you know that neutral atoms of lithium, beryllium, boron and carbon also have 3, 4, 5, and 6 electrons respectively. W ...
... Do you see another of the patterns that Mendeleev and other scientists recognized? ___________ Neutral atoms contain equal numbers of protons (+) and electrons (-). Because of this, you know that neutral atoms of lithium, beryllium, boron and carbon also have 3, 4, 5, and 6 electrons respectively. W ...
SOL Review Station: Equipment, Accuracy, Precision and Lab Safety
... 1. What is formed if you change the following in an atom? a. Protons ...
... 1. What is formed if you change the following in an atom? a. Protons ...
noble gases
... Hydrogen is a unique element (yellow). It’s most common isotope has only a single proton and no neutron in its nucleus. Hydrogen doesn’t have much in common with the alkali metals. It’s a colourless, odourless, tasteless, highly flammable gas. Almost all of Earth’s hydrogen exists in combination wit ...
... Hydrogen is a unique element (yellow). It’s most common isotope has only a single proton and no neutron in its nucleus. Hydrogen doesn’t have much in common with the alkali metals. It’s a colourless, odourless, tasteless, highly flammable gas. Almost all of Earth’s hydrogen exists in combination wit ...
UNIT 3 –TEST REVIEW 1 Atoms of which of the
... H Phosphorus is more like silicon than it is like nitrogen. J Aluminum is smaller than carbon. ...
... H Phosphorus is more like silicon than it is like nitrogen. J Aluminum is smaller than carbon. ...
Standard EPS Shell Presentation
... gases or liquids in their pure form. Fluorine (F), chlorine (Cl), and bromine (Br) form salts when the bond with alkali metals. ...
... gases or liquids in their pure form. Fluorine (F), chlorine (Cl), and bromine (Br) form salts when the bond with alkali metals. ...
Mr. Trachtenberg`s Big Chemistry Test Review Part I of III 20pts
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
Unit 4 Review - Davis
... Modern Periodic Law – The properties of the elements are a periodic function of their atomic numbers. The statement that the physical and chemical properties of the elements repeat in a regular pattern when they are arranged in order of increasing atomic number is known as the periodic law. Octet Ru ...
... Modern Periodic Law – The properties of the elements are a periodic function of their atomic numbers. The statement that the physical and chemical properties of the elements repeat in a regular pattern when they are arranged in order of increasing atomic number is known as the periodic law. Octet Ru ...
File
... Not all carbon atoms are carbon-12 1% of carbon atoms have 7 neutrons. Mass number of these is 13. One more naturally occurring form of carbon atom and its mass number is 14. ...
... Not all carbon atoms are carbon-12 1% of carbon atoms have 7 neutrons. Mass number of these is 13. One more naturally occurring form of carbon atom and its mass number is 14. ...
Periodic table
... An element’s chemical properties are related to the number of electrons in their outside shell ...
... An element’s chemical properties are related to the number of electrons in their outside shell ...
Periodic Table and Atomic Structure Summary
... Astatine is a radioactive element that does not occur naturally on Earth. Like hydrogen, nitrogen and oxygen, the halogens are diatomic molecules. This means they exist in a molecule of two atoms. This means that we can write a chemical formula for them: F2, Cl2, Br2, I2, At2. ...
... Astatine is a radioactive element that does not occur naturally on Earth. Like hydrogen, nitrogen and oxygen, the halogens are diatomic molecules. This means they exist in a molecule of two atoms. This means that we can write a chemical formula for them: F2, Cl2, Br2, I2, At2. ...
22 diatomic molecules
... hydrogen chloride, HCl (one carbon atom and one chlorine atom), and carbon monoxide, CO, (one carbon atom and one oxygen atom). Certain elements normally exist as diatomic molecules. Since diatomic molecules contain two atoms, the chemical formula for an element that is made up of diatomic molecules ...
... hydrogen chloride, HCl (one carbon atom and one chlorine atom), and carbon monoxide, CO, (one carbon atom and one oxygen atom). Certain elements normally exist as diatomic molecules. Since diatomic molecules contain two atoms, the chemical formula for an element that is made up of diatomic molecules ...
Unit 3 Practice Test
... Match each description in Column B with the correct term in Column A. Write the letter of the correct description on the line. Column A ...
... Match each description in Column B with the correct term in Column A. Write the letter of the correct description on the line. Column A ...
The Periodic Table
... • The horizontal rows of elements are called Periods • The period tells the number of shells in the atom of an element • The 1st period has 2 elements H and He. • The 2nd period goes from Li to Ne. • The 3rd period goes from Na to Ar. ...
... • The horizontal rows of elements are called Periods • The period tells the number of shells in the atom of an element • The 1st period has 2 elements H and He. • The 2nd period goes from Li to Ne. • The 3rd period goes from Na to Ar. ...
6-Getting to Know the Periodic Table
... 1) Using blue ink, write in the group numbers of each group below. Label the following families: Alkali Metals, Alkaline Earth Metals, Halogens and Noble Gases. 2) Using red ink, show the lewis dot structure for groups 1-2, 13-18. 3) Using black ink OR pencil, write in the period number for each per ...
... 1) Using blue ink, write in the group numbers of each group below. Label the following families: Alkali Metals, Alkaline Earth Metals, Halogens and Noble Gases. 2) Using red ink, show the lewis dot structure for groups 1-2, 13-18. 3) Using black ink OR pencil, write in the period number for each per ...
- sartep.com
... c. Si, He, Cs, Mg d. Mg, Si, Cs, He 6 .Which diagram to the right is the Lewis electron dot diagram for phosphorous? a. A b. B c. C d. D 7. From left to right across a period, what change is occurring within the atomic nuclei? a. A proton is gained. _ b. An electron is gained. c. A neutron is lost. ...
... c. Si, He, Cs, Mg d. Mg, Si, Cs, He 6 .Which diagram to the right is the Lewis electron dot diagram for phosphorous? a. A b. B c. C d. D 7. From left to right across a period, what change is occurring within the atomic nuclei? a. A proton is gained. _ b. An electron is gained. c. A neutron is lost. ...
A guided tour of the periodic table The periodic table groups
... What is the mass number? ………………………………………………………………………………………..……………. If fluorine has 9 p and 10 n, then the mass number of flurorine = ……………………………………. If oxygen has 8p and 8 n, then the mass number of oxygen = …………………………………. Atoms of an element always have the same atomic number, but they can have dif ...
... What is the mass number? ………………………………………………………………………………………..……………. If fluorine has 9 p and 10 n, then the mass number of flurorine = ……………………………………. If oxygen has 8p and 8 n, then the mass number of oxygen = …………………………………. Atoms of an element always have the same atomic number, but they can have dif ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.