The Periodic Table - Mr Linseman`s wiki
... are soluble in water (group 1) Alkaline earth metals: shiny, silvery metals, form compounds that are often insoluble in water (group 2) Halogens: poisonous, react readily with alkali metals (group 17) Noble gases: do not form compounds (have a full outer shell) (group 18) Periods: The rows of ...
... are soluble in water (group 1) Alkaline earth metals: shiny, silvery metals, form compounds that are often insoluble in water (group 2) Halogens: poisonous, react readily with alkali metals (group 17) Noble gases: do not form compounds (have a full outer shell) (group 18) Periods: The rows of ...
Periodic Trends
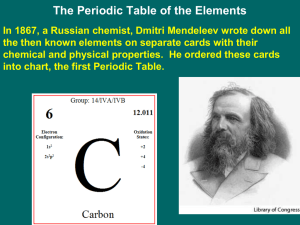
... Periodic Law – there is a periodic repetition of physical and chemical properties when elements are arranged by increasing atomic Some of Medeleev’s work (1869) number ...
... Periodic Law – there is a periodic repetition of physical and chemical properties when elements are arranged by increasing atomic Some of Medeleev’s work (1869) number ...
Physical properties
... substance is not shiny a physical property of metals that allows them to be hammered into different shapes a physical property of metals that allows them to be drawn out into a wire a physical property that allows substances to break or shatter easily a physical property of substances that allows he ...
... substance is not shiny a physical property of metals that allows them to be hammered into different shapes a physical property of metals that allows them to be drawn out into a wire a physical property that allows substances to break or shatter easily a physical property of substances that allows he ...
Chapter6
... For positive ions, charge numbers increase as more electrons are lost from the atom. The electrostatic force is greater for smaller numbers of electrons which decreases the ionic radius. For negative ions, as the charge number increases, so does the number of electrons. Electrostatic forces decrease ...
... For positive ions, charge numbers increase as more electrons are lost from the atom. The electrostatic force is greater for smaller numbers of electrons which decreases the ionic radius. For negative ions, as the charge number increases, so does the number of electrons. Electrostatic forces decrease ...
PPT Periodic Families from Class
... • We will be describing elements according to their reactivity. • Elements that are reactive bond easily with other elements to make compounds. • Some elements are only found in nature bonded with other elements. • What makes an element reactive? • An incomplete valence electron shell. • All atoms ( ...
... • We will be describing elements according to their reactivity. • Elements that are reactive bond easily with other elements to make compounds. • Some elements are only found in nature bonded with other elements. • What makes an element reactive? • An incomplete valence electron shell. • All atoms ( ...
Chemistry
... volume of a material. The formula for calculating density is density = mass divided by volume, D= m/v. The common units of density are grams per cubic centimeter, g/cm or grams per unit milliliter, g/ml. Compound – when two or more elements combine chemically and form a new substance. Compounds have ...
... volume of a material. The formula for calculating density is density = mass divided by volume, D= m/v. The common units of density are grams per cubic centimeter, g/cm or grams per unit milliliter, g/ml. Compound – when two or more elements combine chemically and form a new substance. Compounds have ...
Review: Atomic structure/Periodic Table
... Things you should be able to do: Describe the contribution of different scientists to our understanding of the atom by describing their model of the atom Compare and contrast older models of the atom to the current quantum theory. Read/interpret information from the periodic table (atomic mass ...
... Things you should be able to do: Describe the contribution of different scientists to our understanding of the atom by describing their model of the atom Compare and contrast older models of the atom to the current quantum theory. Read/interpret information from the periodic table (atomic mass ...
Pre-class Activity 12/18
... For positive ions, charge numbers increase as more electrons are lost from the atom. The electrostatic force is greater for smaller numbers of electrons which decreases the ionic radius. For negative ions, as the charge number increases, so does the number of electrons. Electrostatic forces decrease ...
... For positive ions, charge numbers increase as more electrons are lost from the atom. The electrostatic force is greater for smaller numbers of electrons which decreases the ionic radius. For negative ions, as the charge number increases, so does the number of electrons. Electrostatic forces decrease ...
THE PERIODIC TABLE
... B. Multiple Choice Choose the best answer and write its letter on the line. ________ 11. In the periodic table, there is a periodic pattern in the physical and chemical properties of elements when they are arranged in order of a. increasing atomic mass. b. increasing electronegativity. c. increasing ...
... B. Multiple Choice Choose the best answer and write its letter on the line. ________ 11. In the periodic table, there is a periodic pattern in the physical and chemical properties of elements when they are arranged in order of a. increasing atomic mass. b. increasing electronegativity. c. increasing ...
THE PERIODIC TABLE
... B. Multiple Choice Choose the best answer and write its letter on the line. ________ 11. In the periodic table, there is a periodic pattern in the physical and chemical properties of elements when they are arranged in order of a. increasing atomic mass. b. increasing electronegativity. c. increasing ...
... B. Multiple Choice Choose the best answer and write its letter on the line. ________ 11. In the periodic table, there is a periodic pattern in the physical and chemical properties of elements when they are arranged in order of a. increasing atomic mass. b. increasing electronegativity. c. increasing ...
2.2 Periodic Chart
... distinctive colours (Ne is reddish) when electricity is passed through them. Their ion charges of zero indicate that they do not form charged ions. ...
... distinctive colours (Ne is reddish) when electricity is passed through them. Their ion charges of zero indicate that they do not form charged ions. ...
How are properties of atoms used to organize elements into the
... • represents the number of energy levels that contain electrons • FAMILES – columns or groups on the periodic table (up and down) • represents the number of electrons in the outermost energy level • Atoms in the same families have the same number of electrons in their outer energy level. • This char ...
... • represents the number of energy levels that contain electrons • FAMILES – columns or groups on the periodic table (up and down) • represents the number of electrons in the outermost energy level • Atoms in the same families have the same number of electrons in their outer energy level. • This char ...
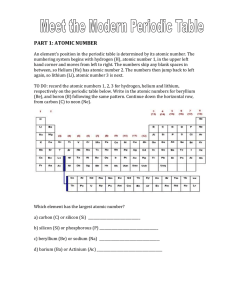
PART 1: ATOMIC NUMBER - hrsbstaff.ednet.ns.ca
... 2. Find all of the gasses in the periodic table and with a black pencil crayon, outline each of them on the above table. 3. In the table below, indicate which gasses are located in each of the periods. Your table will not have elements in every blank space. ...
... 2. Find all of the gasses in the periodic table and with a black pencil crayon, outline each of them on the above table. 3. In the table below, indicate which gasses are located in each of the periods. Your table will not have elements in every blank space. ...
elements-ppt - WordPress.com
... Introduction to the Periodic Table Atomic Number ● Symbol ● Atomic Weight Element ● Compound ● Mixture ...
... Introduction to the Periodic Table Atomic Number ● Symbol ● Atomic Weight Element ● Compound ● Mixture ...
Study Guide Answers
... elements in the same group have similar chemical and physical properties, What are families of the periodic table? There are 18 Families ( or groups), these are the vertical columns; elements in the same group have similar chemical and physical properties, What are at least 3 characteristics for: Al ...
... elements in the same group have similar chemical and physical properties, What are families of the periodic table? There are 18 Families ( or groups), these are the vertical columns; elements in the same group have similar chemical and physical properties, What are at least 3 characteristics for: Al ...
The Periodic Table
... Hydrogen is a diatomic, reactive gas. Hydrogen reacts violently with oxygen. The hot water vapor that forms as a result pushed the space shuttle into orbit. Placed above the group 1 elements because it has only 1 electron in it’s valance shell and can give one away Properties are more like atoms of ...
... Hydrogen is a diatomic, reactive gas. Hydrogen reacts violently with oxygen. The hot water vapor that forms as a result pushed the space shuttle into orbit. Placed above the group 1 elements because it has only 1 electron in it’s valance shell and can give one away Properties are more like atoms of ...
Name________________________ Period____ Date
... 6. What does “Malleable” mean? Can be hammered into flat sheets a. What does “Ductile” mean? Can be pulled into a wire shape 7. What is a metalloid? Along zigzag line of table, have properties of metals and nonmetals. 8. Elements that belong to the same group have _similar_ properties. 9. What infor ...
... 6. What does “Malleable” mean? Can be hammered into flat sheets a. What does “Ductile” mean? Can be pulled into a wire shape 7. What is a metalloid? Along zigzag line of table, have properties of metals and nonmetals. 8. Elements that belong to the same group have _similar_ properties. 9. What infor ...
GO 3_3 The Periodic Table
... They react when exposed to air or water As you move down the group, reactivity increases. Lithium ...
... They react when exposed to air or water As you move down the group, reactivity increases. Lithium ...
Periodic Table PP revised 2014
... • Very stable (not reactive) • A few compounds contain the larger noble gases ...
... • Very stable (not reactive) • A few compounds contain the larger noble gases ...
L2: The Atom, Standard Notation and Borh
... Therefore the number of neutrons is? ________________________________ Question 1: In standard notation nitrogen would be written as How many protons, electrons and neutrons does nitrogen have? #p+ = #e-= #no= ...
... Therefore the number of neutrons is? ________________________________ Question 1: In standard notation nitrogen would be written as How many protons, electrons and neutrons does nitrogen have? #p+ = #e-= #no= ...
Atomic Structure and Periodic Table Worksheet
... ___________________. When water is heated to a vapor, it becomes a ___________________ known as steam. The state in which water is found is determines by the ___________________. Elements are the basic substances from which all other substances are made. They cannot be broken up chemically into simp ...
... ___________________. When water is heated to a vapor, it becomes a ___________________ known as steam. The state in which water is found is determines by the ___________________. Elements are the basic substances from which all other substances are made. They cannot be broken up chemically into simp ...
Families of Elements
... Elements in group IA of the periodic table, with the exception of hydrogen Have one electron in their outer energy levels Are the most chemically active of all metals (meaning an element readily combines with other substances to form compounds) NEVER found in pure form A way to identify al ...
... Elements in group IA of the periodic table, with the exception of hydrogen Have one electron in their outer energy levels Are the most chemically active of all metals (meaning an element readily combines with other substances to form compounds) NEVER found in pure form A way to identify al ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.