Chemistry Study Cards Chapter 5 (3-2) The length of each period in
... Which is the best reason that the atomic radius generally increases with atomic number in each group of elements? ...
... Which is the best reason that the atomic radius generally increases with atomic number in each group of elements? ...
The Periodic Table notes
... noble gas family. These elements do not interact with other elements and they prefer to be alone. They all have 8 valence electrons. ...
... noble gas family. These elements do not interact with other elements and they prefer to be alone. They all have 8 valence electrons. ...
Physical Science Chapter 18 Notes
... The atom and the periodic table o Group: Also All o Period: There are o Electron cloud structure In a neutral atom, The electrons in The lowest energy is Valence electrons: This determines The elements o Energy levels: ...
... The atom and the periodic table o Group: Also All o Period: There are o Electron cloud structure In a neutral atom, The electrons in The lowest energy is Valence electrons: This determines The elements o Energy levels: ...
Introduction To The Periodic Table Of The Elements
... React rapidly when exposed to air and water ...
... React rapidly when exposed to air and water ...
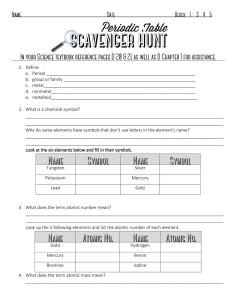
1. Define: a. Period b. group or family
... _________________________________________________________________________________ _________________________________________________________________________________ Why do some elements have symbols that don’t use letters in the element’s name? ________________________________________________________ ...
... _________________________________________________________________________________ _________________________________________________________________________________ Why do some elements have symbols that don’t use letters in the element’s name? ________________________________________________________ ...
Atomic Theory and the Periodic Table PLO`s
... Identify the major divisions within the periodic table including the names and locations of each family of elements. ...
... Identify the major divisions within the periodic table including the names and locations of each family of elements. ...
Periodic Table and Elements Review
... 8.) Two approximate diagrams of periodic tables are shown below. Provide the letter that best describes each statement below: ...
... 8.) Two approximate diagrams of periodic tables are shown below. Provide the letter that best describes each statement below: ...
Study Guide Atoms and Periodic Table TEST Nov 21st
... Introduction to The Periodic table, Metals, Nonmetals and metalloids, Periodic table families You need to know: 1. The scientists responsible for developing and arranging the periodic table and how they arranged it. 2. Difference between a period and group on the periodic table 3. Can you locate an ...
... Introduction to The Periodic table, Metals, Nonmetals and metalloids, Periodic table families You need to know: 1. The scientists responsible for developing and arranging the periodic table and how they arranged it. 2. Difference between a period and group on the periodic table 3. Can you locate an ...
HonorsCh6PracticeTest14
... Match each description in Column B with the correct term in Column A .Write the letter of the correct description on the line. Column A ...
... Match each description in Column B with the correct term in Column A .Write the letter of the correct description on the line. Column A ...
The Periodic Table Worksheet
... Answers to the worksheet 1. The periodic table is defined as an organization of the elements in order of increasing atomic number and grouped according to similar chemical properties and similar electron arrangements. 2. Elements are substances that cannot be broken down into simpler stuff by any c ...
... Answers to the worksheet 1. The periodic table is defined as an organization of the elements in order of increasing atomic number and grouped according to similar chemical properties and similar electron arrangements. 2. Elements are substances that cannot be broken down into simpler stuff by any c ...
The Periodic Table Memorize which elements are gases and
... a.) the reactivity of metals increases down a family and decreases to the right across the table b.) the reactivity of non-metals decreases down a family and increases to the right across a period c.) noble gases are mostly unreactive e.) Fluorine is the most reactive non metal D. Describe mass numb ...
... a.) the reactivity of metals increases down a family and decreases to the right across the table b.) the reactivity of non-metals decreases down a family and increases to the right across a period c.) noble gases are mostly unreactive e.) Fluorine is the most reactive non metal D. Describe mass numb ...
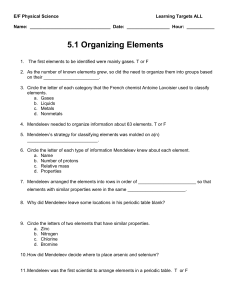
E/F Physical Science Learning Targets ALL Name: Date: Hour
... 7. Most elements exist as a mixture of two or more isotopes. T or F ...
... 7. Most elements exist as a mixture of two or more isotopes. T or F ...
periodictrendsss - rlsciencecurriculum
... seven horizontal rows called periods. Each vertical column in the table contains elements with similar properties. These are called groups or families. The alkali metal family for example consists of the six elements in the first column at the left side of the table. These elements are all highly re ...
... seven horizontal rows called periods. Each vertical column in the table contains elements with similar properties. These are called groups or families. The alkali metal family for example consists of the six elements in the first column at the left side of the table. These elements are all highly re ...
Organization & Characteristics of the Periodic Table
... appearance and are soft enough to cut with a knife Extremely reactive so aren’t found as ...
... appearance and are soft enough to cut with a knife Extremely reactive so aren’t found as ...
Define the following: Electronegativity
... 21. How does atomic radius change from left to right across a period in the periodic table? decreases 22. What element in the first period has the largest atomic radius? Hydrogen 23. What is the charge of a cation? + Anion? 24. What is the element with the lowest electronegativity value? Cesium 25. ...
... 21. How does atomic radius change from left to right across a period in the periodic table? decreases 22. What element in the first period has the largest atomic radius? Hydrogen 23. What is the charge of a cation? + Anion? 24. What is the element with the lowest electronegativity value? Cesium 25. ...
Chapter 5: Atomic Structure and The Periodic Table
... Explain how the atomic theory has changed over time. Identify the contributions of Democritus, Dalton, Thomson, Rutherford, Bohr, Schrodinger and Heisenberg ...
... Explain how the atomic theory has changed over time. Identify the contributions of Democritus, Dalton, Thomson, Rutherford, Bohr, Schrodinger and Heisenberg ...
Chapter 4- The Periodic Table Section 1
... Transition metals- one of the metals that can use the inner shell before using the outer shell in a bond ...
... Transition metals- one of the metals that can use the inner shell before using the outer shell in a bond ...
Name Chemistry Midterm Review Chapter # 2
... 9. Atomic radii tends to ______________ across a period, from left to right on the PT. Atomic radii tends to ______________ down a family. 10. Electronegativity and Ionization energy, both, tend to __________ across a period moving left to right on the PT. Electronegativity and Ionization energy ten ...
... 9. Atomic radii tends to ______________ across a period, from left to right on the PT. Atomic radii tends to ______________ down a family. 10. Electronegativity and Ionization energy, both, tend to __________ across a period moving left to right on the PT. Electronegativity and Ionization energy ten ...
Review of Periodic Trends
... 11. As one moves from left to right ( → ) within a period across the periodic table, the electronegativity of the elements encountered tends to: A. ...
... 11. As one moves from left to right ( → ) within a period across the periodic table, the electronegativity of the elements encountered tends to: A. ...
How Atoms Differ (Section 4.3) part 1
... nuclear charge, so the atom is neutral? • So how many electrons • Atomic number = number of protons = number of electrons • How many electons are in an atom of: – Hydrogen? – Lead? – Chlorine? ...
... nuclear charge, so the atom is neutral? • So how many electrons • Atomic number = number of protons = number of electrons • How many electons are in an atom of: – Hydrogen? – Lead? – Chlorine? ...
Name
... An element is a substance that contains only __________ kind of atom. All atoms of an element have the same number of __________________________, also their atomic number. At this time we know more than _________ elements, but only 90 of these occur in nature. Some familiar natural elements are : __ ...
... An element is a substance that contains only __________ kind of atom. All atoms of an element have the same number of __________________________, also their atomic number. At this time we know more than _________ elements, but only 90 of these occur in nature. Some familiar natural elements are : __ ...
Section 15.1
... gases or liquids in their pure form. Fluorine (F), chlorine (Cl), and bromine (Br) form salts when the bond with alkali metals. ...
... gases or liquids in their pure form. Fluorine (F), chlorine (Cl), and bromine (Br) form salts when the bond with alkali metals. ...
Chemistry Fall Final Review Worksheet Part 1
... 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. As a basis for understanding this concept: a. Students know how to relate the position of an element in the periodic ...
... 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. As a basis for understanding this concept: a. Students know how to relate the position of an element in the periodic ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.