Unit 10: Chemical Periodicity
... d sublevel generally contain electrons 20. _______ group d. an element whose outermost s and nearby f sublevel generally contain electrons e. a vertical column on the periodic table f. an element whose outermost s and p sublevels are only partially filled Part D Short Answer 21. List the outer elect ...
... d sublevel generally contain electrons 20. _______ group d. an element whose outermost s and nearby f sublevel generally contain electrons e. a vertical column on the periodic table f. an element whose outermost s and p sublevels are only partially filled Part D Short Answer 21. List the outer elect ...
noble gases
... Hydrogen is a unique element (yellow). It’s most common isotope has only a single proton and no neutron in its nucleus. Hydrogen doesn’t have much in common with the alkali metals. It’s a colourless, odourless, tasteless, highly flammable gas. Almost all of Earth’s hydrogen exists in combination wit ...
... Hydrogen is a unique element (yellow). It’s most common isotope has only a single proton and no neutron in its nucleus. Hydrogen doesn’t have much in common with the alkali metals. It’s a colourless, odourless, tasteless, highly flammable gas. Almost all of Earth’s hydrogen exists in combination wit ...
atomic number
... The first group is called the alkali metals The second group is the alkaline-earth metals Groups 3-12 are called the transition metals Groups 13-15 are not named Group 16 are the chalcogens Group 17 are the halogens Group 18 are the noble gases and are basically inert ...
... The first group is called the alkali metals The second group is the alkaline-earth metals Groups 3-12 are called the transition metals Groups 13-15 are not named Group 16 are the chalcogens Group 17 are the halogens Group 18 are the noble gases and are basically inert ...
Structure of Atoms/Periodic Table Review 1. Shade in location of the
... 20. Why are atoms neutral? They have the _________ __________ of protons and neutrons. 22. What are the limitations of the Bohr model? ____________ do not orbit the nucleus like planets orbit the sun. The ___________ does not represent the actual size of an atom. 23. What determines an element’s ide ...
... 20. Why are atoms neutral? They have the _________ __________ of protons and neutrons. 22. What are the limitations of the Bohr model? ____________ do not orbit the nucleus like planets orbit the sun. The ___________ does not represent the actual size of an atom. 23. What determines an element’s ide ...
Chapter 6 Periodic law- states that when the elements are arranged
... Transition metal- A type of group B element that is contained in the d-block of the periodic table and, with some exceptions, is characterized by a filled outermost s orbital of energy level n, and filled or partially filled d orbitals of energy level n-1 Inner transition metal- A type of group B el ...
... Transition metal- A type of group B element that is contained in the d-block of the periodic table and, with some exceptions, is characterized by a filled outermost s orbital of energy level n, and filled or partially filled d orbitals of energy level n-1 Inner transition metal- A type of group B el ...
Periodic Table cloze activity.
... atom, atomic number, column, element, gold, inert, Mendeleev, metals, nonmetals, periodic, properties, symbol All matter is composed of various elements. An _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the ___ ...
... atom, atomic number, column, element, gold, inert, Mendeleev, metals, nonmetals, periodic, properties, symbol All matter is composed of various elements. An _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the ___ ...
Lecture Guide Molecules • Ions • Atoms I. Development of the Atomic
... How does the suffix of an oxy-anion change when oxygen are removed or added? ...
... How does the suffix of an oxy-anion change when oxygen are removed or added? ...
Name - TeacherWeb
... 10. Circle the letter of each sentence that is true about a carbon-12 atom. a. It has 6 protons and 6 neutrons. b. Scientists assigned a mass of 6 atomic mass units to the carbon-12 atom. c. It is used as a standard for comparing the masses of atoms. d. An atomic mass unit is defined as one twelfth ...
... 10. Circle the letter of each sentence that is true about a carbon-12 atom. a. It has 6 protons and 6 neutrons. b. Scientists assigned a mass of 6 atomic mass units to the carbon-12 atom. c. It is used as a standard for comparing the masses of atoms. d. An atomic mass unit is defined as one twelfth ...
Elements and the Periodic Table Section One
... Malleable: a term used to describe material that can be hammered or rolled into shape (pg. ...
... Malleable: a term used to describe material that can be hammered or rolled into shape (pg. ...
4-3 Families of Elements
... a. The alkali metals are very reactive i. Very reactive because they have one valence electron that can easily be removed to form a positive ion ii. Because they are so reactive, they are not found in nature as elements--they combine with other elements to form compounds o ...
... a. The alkali metals are very reactive i. Very reactive because they have one valence electron that can easily be removed to form a positive ion ii. Because they are so reactive, they are not found in nature as elements--they combine with other elements to form compounds o ...
2.5-The Periodic Table
... The periodic table is arranged into: - rows – called periods. The trend within a period is increasing atomic mass. - Columns – called groups or families. The trend within a group is the elements ...
... The periodic table is arranged into: - rows – called periods. The trend within a period is increasing atomic mass. - Columns – called groups or families. The trend within a group is the elements ...
Review: Atomic structure/Periodic Table
... Determine the number of protons, neutrons and electrons for any element Relate the organization of the Periodic Table to the arrangement of electrons within an atom Explain the relationship between atomic mass and isotopes of an element Draw/analyze and interpret Bohr models for elements 1-1 ...
... Determine the number of protons, neutrons and electrons for any element Relate the organization of the Periodic Table to the arrangement of electrons within an atom Explain the relationship between atomic mass and isotopes of an element Draw/analyze and interpret Bohr models for elements 1-1 ...
Elements and the Periodic Table Practice Test
... 9. There exists several _____________ of argon, such as Ar-36, Ar-38, and Ar-40. 10.Yet the mass for argon on the periodic table is 39.948 atomic mass units and not 36, 38, or 40. Explain why. ...
... 9. There exists several _____________ of argon, such as Ar-36, Ar-38, and Ar-40. 10.Yet the mass for argon on the periodic table is 39.948 atomic mass units and not 36, 38, or 40. Explain why. ...
Reading the Periodic Table
... •Have properties of both metals and nonmetals. •Some of the metalloids, such as silicon and germanium, are semi-conductors. This means that they can carry an electrical charge under special conditions. This property makes metalloids useful in computers and calculators ...
... •Have properties of both metals and nonmetals. •Some of the metalloids, such as silicon and germanium, are semi-conductors. This means that they can carry an electrical charge under special conditions. This property makes metalloids useful in computers and calculators ...
Name Period
... 6. A(n) _______________ transmits heat and electricity easily. 7. A material that is _________________ can be drawn into a wire. 8. ____________________ is the ease and speed with which an element combines with other elements and compounds. 9. A(n) ______________________ is a mixture of metals. ...
... 6. A(n) _______________ transmits heat and electricity easily. 7. A material that is _________________ can be drawn into a wire. 8. ____________________ is the ease and speed with which an element combines with other elements and compounds. 9. A(n) ______________________ is a mixture of metals. ...
Periodic Table and Elements Review
... 15.) The first, second, third, and fourth ionization energies are 578, 1817, 2745, and 11,577 kJ/mol, respectively. What is probably the charge of an ion of this element, and why? ...
... 15.) The first, second, third, and fourth ionization energies are 578, 1817, 2745, and 11,577 kJ/mol, respectively. What is probably the charge of an ion of this element, and why? ...
Name: Date: Period: ______ Graphing Periodic Trends Purpose:To
... Jj is considered to be the “last” (highest atomic number) naturally-occurring element; it forms beautiful crystals and is used in that pink stomach-ache medicine that makes your tongue turn black. ...
... Jj is considered to be the “last” (highest atomic number) naturally-occurring element; it forms beautiful crystals and is used in that pink stomach-ache medicine that makes your tongue turn black. ...
Atoms, Bonding, and the Periodic Table Electron Dot Diagrams
... The valence electrons of an atom are shown as dots around the symbol of the element. Complete the electron dot diagram for neon. ...
... The valence electrons of an atom are shown as dots around the symbol of the element. Complete the electron dot diagram for neon. ...
- Priddy ISD
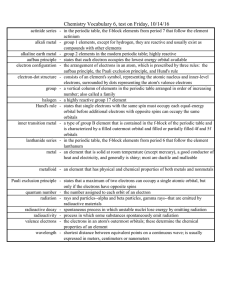
... Chemistry Vocabulary 6, test on Friday, 10/14/16 actinide series - in the periodic table, the f-block elements from period 7 that follow the element actinium alkali metal - group 1 elements, except for hydrogen, they are reactive and usually exist as compounds with other elements alkaline earth meta ...
... Chemistry Vocabulary 6, test on Friday, 10/14/16 actinide series - in the periodic table, the f-block elements from period 7 that follow the element actinium alkali metal - group 1 elements, except for hydrogen, they are reactive and usually exist as compounds with other elements alkaline earth meta ...
Chapter 12 The Periodic Table
... silvery in their pure form and are highly reactive. This group includes the elements lithium (Li), sodium (Na), and potassium (K). ...
... silvery in their pure form and are highly reactive. This group includes the elements lithium (Li), sodium (Na), and potassium (K). ...
Atomic and Molecular Structure – Standard 1 Review
... 1a.3 What does an element’s Mass Number (Atomic Mass) tell you about that element? ...
... 1a.3 What does an element’s Mass Number (Atomic Mass) tell you about that element? ...
ELEMENTS and THEIR PROPERTIES
... The inner Transition metals- seem disconnected from the rest of the periodic table • The Lathanides- include lanthanum, cerium, praseodymium, amarium, europium, gadolinium, and terbium. • The Actinides- all are radioactive and unstable; uranium is the best ...
... The inner Transition metals- seem disconnected from the rest of the periodic table • The Lathanides- include lanthanum, cerium, praseodymium, amarium, europium, gadolinium, and terbium. • The Actinides- all are radioactive and unstable; uranium is the best ...
Atomic Theory and the Periodic Table Video Questions
... What did Meneleev order the elements by? What did Moseley order the elements by? What is the chemical symbol for magnesium? Where is one place the compounds of potassium are found? What is used in a pencil for writing? What do we call atoms of the same element with different numbers of neutrons? Wha ...
... What did Meneleev order the elements by? What did Moseley order the elements by? What is the chemical symbol for magnesium? Where is one place the compounds of potassium are found? What is used in a pencil for writing? What do we call atoms of the same element with different numbers of neutrons? Wha ...
Period 3 element
A period 3 element is one of the chemical elements in the third row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when the periodic table skips a row and a chemical behaviour begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. Note that there is a 3d orbital, but it is not filled until Period 4, such giving the period table its characteristic shape of ""two rows at a time"". All of the period 3 elements occur in nature and have at least one stable isotope.